Efficiency of CIN2+ Detection by Thyrotropin-Releasing Hormone (TRH) Site-Specific Methylation
- PMID: 37766209
- PMCID: PMC10535538
- DOI: 10.3390/v15091802
Efficiency of CIN2+ Detection by Thyrotropin-Releasing Hormone (TRH) Site-Specific Methylation
Abstract
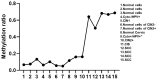
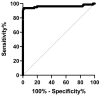
Cervical cancer screening typically involves a Pap smear combined with high-risk human papillomavirus (hr-HPV) detection. Women with hr-HPV positivity but normal cytology, as well as those with precancerous abnormal cytology, such as low-grade squamous intraepithelial lesions (LSIL) and high-grade SIL (HSIL), are referred for colposcopy and histology examination to identify abnormal lesions, such as cervical intraepithelial neoplasia (CIN) and cervical cancer. However, in order to enhance the accuracy of detection, bioinformatics analysis of a microarray database was performed, which identified cg01009664, a methylation marker of the thyrotropin-releasing hormone (TRH). Consequently, a real-time PCR assay was developed to distinguish CIN2+ (CIN2, CIN3, and cervical cancer) from CIN2- (CIN1 and normal cervical epithelia). The real-time PCR assay utilized specific primers targeting methylated cg01009664 sites, whereas an unmethylated reaction was used to check the DNA quality. A cut-off value for the methylated reaction of Ct < 33 was established, resulting in improved precision in identifying CIN2+. In the first cohort group, the assay demonstrated a sensitivity of 93.7% and a specificity of 98.6%. In the cytology samples identified as atypical squamous cells of undetermined significance (ASC-US) and LSIL, the sensitivity and specificity for detecting CIN2+ were 95.0% and 98.9%, respectively. However, when self-collected samples from women with confirmed histology were tested, the sensitivity for CIN2+ detection dropped to 49.15%, while maintaining a specificity of 100%. Notably, the use of clinician-collected samples increased the sensitivity of TRH methylation testing. TRH methylation analysis can effectively identify women who require referral for colposcopy examinations, aiding in the detection of CIN2+.
Keywords: CIN2+; cervical cancer; methylation; thyrotropin-releasing hormone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Role of p16(INK4a) cytology testing as an adjunct to enhance the diagnostic specificity and accuracy in human papillomavirus-positive women within an organized cervical cancer screening program.Acta Cytol. 2012;56(5):506-14. doi: 10.1159/000338979. Epub 2012 Sep 27. Acta Cytol. 2012. PMID: 23075891
-
[Evaluation of CIN2+ /CIN3+ risk of different HPV subtypes infection combined with abnormal cytology status].Zhonghua Zhong Liu Za Zhi. 2018 Mar 23;40(3):232-238. doi: 10.3760/cma.j.issn.0253-3766.2018.03.015. Zhonghua Zhong Liu Za Zhi. 2018. PMID: 29575846 Chinese.
-
[The Clinical Significance of HPV L1/PD-L1 Tests Combined with Colposcopy for Cervical Precancerous Lesions and Cervical Cancer].Sichuan Da Xue Xue Bao Yi Xue Ban. 2021 May;52(3):516-522. doi: 10.12182/20210560105. Sichuan Da Xue Xue Bao Yi Xue Ban. 2021. PMID: 34018374 Free PMC article. Chinese.
-
Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer.Vaccine. 2012 Nov 20;30 Suppl 5:F88-99. doi: 10.1016/j.vaccine.2012.06.095. Vaccine. 2012. PMID: 23199969 Review.
-
Triage of women with minor abnormal cervical cytology: meta-analysis of the accuracy of an assay targeting messenger ribonucleic acid of 5 high-risk human papillomavirus types.Cancer Cytopathol. 2013 Dec;121(12):675-87. doi: 10.1002/cncy.21325. Epub 2013 Jul 23. Cancer Cytopathol. 2013. PMID: 23881840 Review.
Cited by
-
Clinical indications for host-cell DNA methylation markers in cervical screening and management of cervical intraepithelial neoplasia: A review.Tumour Virus Res. 2025 Jun;19:200308. doi: 10.1016/j.tvr.2024.200308. Epub 2024 Dec 16. Tumour Virus Res. 2025. PMID: 39694193 Free PMC article. Review.
-
The current state of DNA methylation biomarkers in self-collected liquid biopsies for the early detection of cervical cancer: a literature review.Infect Agent Cancer. 2024 Dec 18;19(1):62. doi: 10.1186/s13027-024-00623-1. Infect Agent Cancer. 2024. PMID: 39695781 Free PMC article.
References
-
- Vink M.A., Bogaards J.A., van Kemenade F.J., de Melker H.E., Meijer C.J., Berkhof J. Clinical progression of high-grade cervical intraepithelial neoplasia: Estimating the time to preclinical cervical cancer from doubly censored national registry data. Am. J. Epidemiol. 2013;178:1161–1169. doi: 10.1093/aje/kwt077. - DOI - PubMed
-
- Mashburn J., Scharbo-DeHaan M. A clinician’s guide to Pap smear interpretation. Nurse Pract. 1997;22:115–118, 124–127, 130 passim. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

