Targeting FOXP3 Tumor-Intrinsic Effects Using Adenoviral Vectors in Experimental Breast Cancer
- PMID: 37766222
- PMCID: PMC10537292
- DOI: 10.3390/v15091813
Targeting FOXP3 Tumor-Intrinsic Effects Using Adenoviral Vectors in Experimental Breast Cancer
Abstract
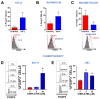
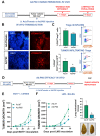
The regulatory T cell master transcription factor, Forkhead box P3 (Foxp3), has been detected in cancer cells; however, its role in breast tumor pathogenesis remains controversial. Here we assessed Foxp3 tumor intrinsic effects in experimental breast cancer using a Foxp3 binder peptide (P60) that impairs Foxp3 nuclear translocation. Cisplatin upregulated Foxp3 expression in HER2+ and triple-negative breast cancer (TNBC) cells. Foxp3 inhibition with P60 enhanced chemosensitivity and reduced cell survival and migration in human and murine breast tumor cells. We also developed an adenoviral vector encoding P60 (Ad.P60) that efficiently transduced breast tumor cells, reduced cell viability and migration, and improved the cytotoxic response to cisplatin. Conditioned medium from transduced breast tumor cells contained lower levels of IL-10 and improved the activation of splenic lymphocytes. Intratumoral administration of Ad.P60 in breast-tumor-bearing mice significantly reduced tumor infiltration of Tregs, delayed tumor growth, and inhibited the development of spontaneous lung metastases. Our results suggest that Foxp3 exerts protumoral intrinsic effects in breast cancer cells and that gene-therapy-mediated blockade of Foxp3 could constitute a therapeutic strategy to improve the response of these tumors to standard treatment.
Keywords: Foxp3; breast cancer; cell penetrating peptide; chemosensitivity; gene therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Targeting Inhibition of Foxp3 by MMP2/9 Sensitive Short Peptide Linked P60 Fusion Protein 6(P60-MMPs) to Enhance Antitumor Immunity.Macromol Biosci. 2020 Jul;20(7):e2000098. doi: 10.1002/mabi.202000098. Epub 2020 May 25. Macromol Biosci. 2020. PMID: 32449306
-
Therapeutic blockade of Foxp3 in experimental breast cancer models.Breast Cancer Res Treat. 2017 Nov;166(2):393-405. doi: 10.1007/s10549-017-4414-2. Epub 2017 Jul 29. Breast Cancer Res Treat. 2017. PMID: 28756536
-
Association of Notch pathway down-regulation with Triple Negative/Basal-like breast carcinomas and high tumor-infiltrating FOXP3+ Tregs.Exp Mol Pathol. 2016 Jun;100(3):460-8. doi: 10.1016/j.yexmp.2016.04.006. Epub 2016 Apr 23. Exp Mol Pathol. 2016. PMID: 27118257
-
FOXP3 regulatory T cells on prognosis of breast cancer.Breast Dis. 2023;42(1):213-218. doi: 10.3233/BD-239002. Breast Dis. 2023. PMID: 37458005 Review.
-
Signalling through FOXP3 as an X-linked tumor suppressor.Int J Biochem Cell Biol. 2010 Nov;42(11):1784-7. doi: 10.1016/j.biocel.2010.07.015. Epub 2010 Aug 1. Int J Biochem Cell Biol. 2010. PMID: 20678582 Free PMC article. Review.
Cited by
-
CD25+FOXP3+CD45RA- regulatory T-cell infiltration as a prognostic biomarker for endometrial carcinoma.BMC Cancer. 2024 Sep 4;24(1):1100. doi: 10.1186/s12885-024-12851-0. BMC Cancer. 2024. PMID: 39232704 Free PMC article.
-
Treg Cell Therapeutic Strategies for Breast Cancer: Holistic to Local Aspects.Cells. 2024 Sep 11;13(18):1526. doi: 10.3390/cells13181526. Cells. 2024. PMID: 39329710 Free PMC article. Review.
-
Intratumoral delivery of immunotherapy to treat breast cancer: current development in clinical and preclinical studies.Front Immunol. 2024 May 13;15:1385484. doi: 10.3389/fimmu.2024.1385484. eCollection 2024. Front Immunol. 2024. PMID: 38803496 Free PMC article. Review.
References
-
- Colamatteo A., Carbone F., Bruzzaniti S., Galgani M., Fusco C., Maniscalco G.T., Di Rella F., de Candia P., De Rosa V. Molecular Mechanisms Controlling Foxp3 Expression in Health and Autoimmunity: From Epigenetic to Post-translational Regulation. Front. Immunol. 2019;10:3136. doi: 10.3389/fimmu.2019.03136. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

