Safety and Immunogenicity of Inactivated Whole Virion COVID-19 Vaccine CoviVac in Clinical Trials in 18-60 and 60+ Age Cohorts
- PMID: 37766235
- PMCID: PMC10537914
- DOI: 10.3390/v15091828
Safety and Immunogenicity of Inactivated Whole Virion COVID-19 Vaccine CoviVac in Clinical Trials in 18-60 and 60+ Age Cohorts
Abstract
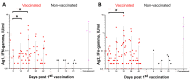
We present the results of a randomized, double-blind, placebo-controlled, multi-center clinical trial phase I/II of the tolerability, safety, and immunogenicity of the inactivated whole virion concentrated purified coronavirus vaccine CoviVac in volunteers aged 18-60 and open multi-center comparative phase IIb clinical trial in volunteers aged 60 years and older. The safety of the vaccine was assessed in 400 volunteers in the 18-60 age cohort who received two doses of the vaccine (n = 300) or placebo (n = 100) and in 200 volunteers in 60+ age cohort all of whom received three doses of the vaccine. The studied vaccine has shown good tolerability and safety. No deaths, serious adverse events (AEs), or other significant AEs related to vaccination have been detected. The most common AE in vaccinated participants was pain at the injection site (p < 0.05). Immunogenicity assessment in stage 3 of Phase II was performed on 167 volunteers (122 vaccinated and 45 in Placebo Group) separately for the participants who were anti-SARS-CoV-2 nAB negative (69/122 in Vaccine Group and 28/45 in Placebo Group) or positive (53/122 in Vaccine Group and 17/45 in Placebo Group) at screening. On Day 42 after the 1st vaccination, the seroconversion rate in participants who were seronegative at screening was 86.9%, with the average geometric mean neutralizing antibody (nAB) titer of 1:20. A statistically significant (p < 0.05) increase in IFN-γ production by peptide-stimulated T-cells was observed at Days 14 and 21 after the 1st vaccination. In participants who were seropositive at screening but had nAB titers below 1:256, the rate of fourfold increase in nAB levels was 85.2%, while in the participants with nAB titers > 1:256, the rate of fourfold increase in nAB levels was below 45%; the participants who were seropositive at screening of the 2nd vaccination did not lead to a significant increase in nAB titers. In conclusion, inactivated vaccine CoviVac has shown good tolerability and safety, with over 85% NT seroconversion rates after complete vaccination course in participants who were seronegative at screening in both age groups: 18-60 and 60+. In participants who were seropositive at screening and had nAB titers below 1:256, a single vaccination led to a fourfold increase in nAB levels in 85.2% of cases. These findings indicate that CoviVac can be successfully used both for primary vaccination in a two-dose regimen and for booster vaccination as a single dose in individuals with reduced neutralizing antibody levels.
Keywords: 18–60 age cohort; 60+ age cohort; COVID-19; inactivated vaccine; neutralizing antibodies; phase I-II clinical trials.
Conflict of interest statement
A.A.I., A.A.S. (Aleksandra A. Siniugina), V.P.V., M.S.D., L.V.G., N.A.K., R.D.T., G.M.I., A.K.K., E.Y.S., E.O.B., A.S.Z., D.V.A., A.N.P., A.A.K. (Anastasia A. Kovpak), L.P.A., Y.V.R., A.A.S. (Anna A. Shishova), Y.Y.I., S.E.S., K.A.C, E.G.I., E.A.K., L.I.K. and I.V.G. are employees at the Chumakov Federal Scientific Center for Research and Development of Immune-and-Biological Products of Russian Academy of Sciences, which developed and manufactured CoviVac.
Figures

References
-
- WHO WHO Coronavirus (COVID-19) Dashboard. WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. [(accessed on 30 May 2023)];2023 Available online: https://covid19.who.int/
-
- Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

