Viral Co-Infection in Bats: A Systematic Review
- PMID: 37766267
- PMCID: PMC10535902
- DOI: 10.3390/v15091860
Viral Co-Infection in Bats: A Systematic Review
Abstract
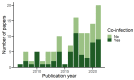
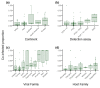
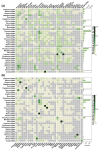
Co-infection is an underappreciated phenomenon in contemporary disease ecology despite its ubiquity and importance in nature. Viruses, and other co-infecting agents, can interact in ways that shape host and agent communities, influence infection dynamics, and drive evolutionary selective pressures. Bats are host to many viruses of zoonotic potential and have drawn increasing attention in their role as wildlife reservoirs for human spillover. However, the role of co-infection in driving viral transmission dynamics within bats is unknown. Here, we systematically review peer-reviewed literature reporting viral co-infections in bats. We show that viral co-infection is common in bats but is often only reported as an incidental finding. Biases identified in our study database related to virus and host species were pre-existing in virus studies of bats generally. Studies largely speculated on the role co-infection plays in viral recombination and few investigated potential drivers or impacts of co-infection. Our results demonstrate that current knowledge of co-infection in bats is an ad hoc by-product of viral discovery efforts, and that future targeted co-infection studies will improve our understanding of the role it plays. Adding to the broader context of co-infection studies in other wildlife species, we anticipate our review will inform future co-infection study design and reporting in bats. Consideration of detection strategy, including potential viral targets, and appropriate analysis methodology will provide more robust results and facilitate further investigation of the role of viral co-infection in bat reservoirs.
Keywords: chiroptera; co-infection; coinfection; review; viral co-infection; viral dynamics; wildlife.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Viney M.E., Graham A.L. Chapter Five-Patterns and Processes in Parasite Co-Infection. In: Rollinson D., editor. Advances in Parasitology. Volume 82. Academic Press; Cambridge, MA, USA: 2013. pp. 321–369. - PubMed
-
- Pantin-Jackwood M.J., Costa-Hurtado M., Miller P.J., Afonso C.L., Spackman E., Kapczynski D.R., Shepherd E., Smith D., Swayne D.E. Experimental co-infections of domestic ducks with a virulent Newcastle disease virus and low or highly pathogenic avian influenza viruses. Veter. Microbiol. 2015;177:7–17. doi: 10.1016/j.vetmic.2015.02.008. - DOI - PMC - PubMed
-
- Davy C.M., Donaldson M.E., Subudhi S., Rapin N., Warnecke L., Turner J.M., Bollinger T.K., Kyle C.J., Dorville N.A.S.-Y., Kunkel E.L., et al. White-nose syndrome is associated with increased replication of a naturally persisting coronaviruses in bats. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-33975-x. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

