Human Rotavirus Replicates in Salivary Glands and Primes Immune Responses in Facial and Intestinal Lymphoid Tissues of Gnotobiotic Pigs
- PMID: 37766270
- PMCID: PMC10534682
- DOI: 10.3390/v15091864
Human Rotavirus Replicates in Salivary Glands and Primes Immune Responses in Facial and Intestinal Lymphoid Tissues of Gnotobiotic Pigs
Abstract
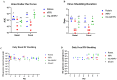
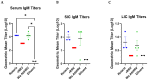
Human rotavirus (HRV) is a leading cause of viral gastroenteritis in children across the globe. The virus has long been established as a pathogen of the gastrointestinal tract, targeting small intestine epithelial cells and leading to diarrhea, nausea, and vomiting. Recently, this classical infection pathway was challenged by the findings that murine strains of rotavirus can infect the salivary glands of pups and dams and transmit via saliva from pups to dams during suckling. Here, we aimed to determine if HRV was also capable of infecting salivary glands and spreading in saliva using a gnotobiotic (Gn) pig model of HRV infection and disease. Gn pigs were orally inoculated with various strains of HRV, and virus shedding was monitored for several days post-inoculation. HRV was shed nasally and in feces in all inoculated pigs. Infectious HRV was detected in the saliva of four piglets. Structural and non-structural HRV proteins, as well as the HRV genome, were detected in the intestinal and facial tissues of inoculated pigs. The pigs developed high IgM antibody responses in serum and small intestinal contents at 10 days post-inoculation. Additionally, inoculated pigs had HRV-specific IgM antibody-secreting cells present in the ileum, tonsils, and facial lymphoid tissues. Taken together, these findings indicate that HRV can replicate in salivary tissues and prime immune responses in both intestinal and facial lymphoid tissues of Gn pigs.
Keywords: gnotobiotic pigs; rotavirus; salivary glands.
Conflict of interest statement
L.L. and M.B. receive cash and equity compensation from GIVAX as employees of the company. L.Y. and J.P. are scientific advisors of GIVAX and have an equity interest in the company. V.P. receives cash compensation for providing consulting services to GIVAX. J.P.’s laboratory receives funding from GIVAX through a sponsored research agreement.
Figures




References
-
- Fields B.N. Fields Virology. Volume 6 Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

