Comparison of Gross Pathology between Classical and Recombinant Lumpy Skin Disease Viruses
- PMID: 37766289
- PMCID: PMC10537798
- DOI: 10.3390/v15091883
Comparison of Gross Pathology between Classical and Recombinant Lumpy Skin Disease Viruses
Abstract
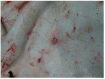
The pathology caused by three different isolates of lumpy skin disease virus, classical field cluster 1.2 strain Dagestan/2015, recombinant vaccine-like cluster 2.1 strain Saratov/2017, and cluster 2.2 strain Udmurtiya/2019, in cattle was compared from experimental infections. The infection of cattle was performed using intravenous administration of 2 mL of 105 TCID50/mL of each specific LSDV. Both classical and recombinant forms of LSDV cause pathological changes in the skin and lymph nodes, as well as the trachea and lungs. Due to circulatory disorders in the affected organs, multiple areas of tissue necrosis were observed, which, with the resurgence of secondary microflora, led to the development of purulent inflammation. Observed pathological changes caused by the recombinant vaccine-like strain Udmurtiya/2019 were characterized by a more pronounced manifestation of the pathoanatomical picture compared to the classical field strains Dagestan/2015 and Saratov/2017. Interestingly, Dagestan/2015 and Udmurtiya/2019 caused damage to the lymph nodes, characterized by serous inflammation and focal purulent lymphadenitis caused by purulent microflora. "Saratov/2017" did not cause pathology in the lymph nodes. All LSDVs were virulent and caused pathology, which was not distinguishable between viruses. This data set will serve as the experimentally validated basis for the comparative examination of novel LSDV strains in gross pathology.
Keywords: experimental bioassays; lumpy skin disease virus; lumpy skin diseases; necropsy; pathology; recombinant virus.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
-
- Sprygin A., Sainnokhoi T., Gombo-Ochir D., Tserenchimed T., Tsolmon A., Byadovskaya O., Ankhanbaatar U., Mazloum A., Korennoy F., Chvala I. Genetic characterization and epidemiological analysis of the first lumpy skin disease virus outbreak in Mongolia, 2021. Transbound. Emerg. Dis. 2022;69:3664–3672. doi: 10.1111/tbed.14736. - DOI - PubMed
-
- Le Goff C., Lamien C.E., Fakhfakh E., Chadeyras A., Aba-Adulugba E., Libeau G., Tuppurainen E., Wallace D.B., Adam T., Silber R., et al. Capripoxvirus G-protein-coupled chemokine receptor: A host-range gene suitable for virus animal origin discrimination. J. Gen. Virol. 2009;90:1967–1977. doi: 10.1099/vir.0.010686-0. - DOI - PubMed
-
- Kononov A., Prutnikov P., Shumilova I., Kononova S., Nesterov A., Byadovskaya O., Pestova Y., Diev V., Sprygin A. Determination of lumpy skin disease virus in bovine meat and offal products following experimental infection. Transbound. Emerg. Dis. 2019;66:1332–1340. doi: 10.1111/tbed.13158. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

