Accuracy of Human Papillomavirus (HPV) Testing on Urine and Vaginal Self-Samples Compared to Clinician-Collected Cervical Sample in Women Referred to Colposcopy
- PMID: 37766295
- PMCID: PMC10537107
- DOI: 10.3390/v15091889
Accuracy of Human Papillomavirus (HPV) Testing on Urine and Vaginal Self-Samples Compared to Clinician-Collected Cervical Sample in Women Referred to Colposcopy
Abstract
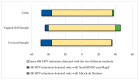
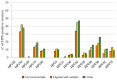
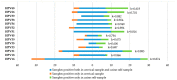
In the context of cervical cancer prevention, where human papillomavirus (HPV) infection is pivotal, HPV testing is replacing Pap Smear in primary screening. This transition offers an opportunity for integrating self-sampling to enhance coverage. We evaluated the accuracy of HPV testing using self-collected urine and vaginal samples, comparing them to physician-collected cervical swabs. From a cohort of 245 women with abnormal cytology, we collected self-sampled vaginal, urine, and clinician-administered cervical specimens. Employing Anyplex™II HPV28 assay, outcomes revealed HPV positivity rates of 75.1% (cervical), 78.4% (vaginal), and 77.1% (urine). Significant, hr-HPV detection concordance was observed between self-taken cervical samples and clinical counterparts (k = 0.898 for vaginal; k = 0.715 for urine). This study extends beyond accuracy, highlighting self-collected sample efficacy in detecting high-grade cervical lesions. The insight underscores self-sampling's role in bolstering participation and aligns with WHO's goal to eliminate cervical cancer by 2030.
Keywords: HPV infection; HPV testing; cervical cancer; low-risk HPV; self-sampling.
Conflict of interest statement
C.E.C. received research support and/or honoraria from BD Diagnostics, Seegene, Arrows Diagnostics, Copan, GeneFirst, and Hiantis. M.M., C.G., M.M., C.G., M.L.D., F.P., B.E.L., R.F. and F.L. declare no conflict of interest.
Figures



References
-
- Ervik M., Lam F., Ferlay J., Mery L., Soerjomataram I., Bray F. Cancer Today. International Agency for Research on Cancer; Lyon, France: 2020.
-
- Bruni L., Albero G., Serrano B., Mena M., Collado J.J., Gómez D., Munoz J., Bosch F.X., de Sanjosé S. Human Papillomavirus and Related Diseases in the World. ICO/IARC Information Centre on HPV; Lyon, France: 2023. Summary Report 10 March 2023.
-
- Aiom-Airtum I. Numeri del Cancro in Italia. Edizione; Treviso, Italy: 2020.
-
- Ronco G., Dillner J., Elfstrom K.M., Tunesi S., Snijders P.J., Arbyn M., Kitchener H., Segnan N., Gilham C., Giorgi-Rossi P., et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet. 2014;383:524–532. doi: 10.1016/S0140-6736(13)62218-7. - DOI - PubMed

