Effect of dolutegravir on folate, vitamin B12 and mean corpuscular volume levels among children and adolescents with HIV: a sub-study of the ODYSSEY randomized controlled trial
- PMID: 37766505
- PMCID: PMC10534059
- DOI: 10.1002/jia2.26174
Effect of dolutegravir on folate, vitamin B12 and mean corpuscular volume levels among children and adolescents with HIV: a sub-study of the ODYSSEY randomized controlled trial
Abstract
Introduction: Dolutegravir-based antiretroviral therapy (ART) is the preferred antiretroviral treatment for children and adolescents living with HIV. A large surveillance study in Botswana previously raised concerns about an association between pre-conception dolutegravir and neural tube defects. Before these concerns were subsequently resolved, we set up a sub-study to look at the effect of dolutegravir on levels of folate and vitamin B12 in children and adolescents within the randomized ODYSSEY trial, as folate and vitamin B12 are known to play a crucial role in neural tube development.
Methods: We conducted the sub-study among Ugandan ODYSSEY participants and compared folate and vitamin B12 between children randomized to dolutegravir-based ART (DTG) and non-dolutegravir-based standard-of-care treatment (SOC). Plasma folate was measured at enrolment and week 4 on stored samples; in addition, plasma and red blood cell (RBC) folate and vitamin B12 were assayed at week ≥96 in prospectively collected samples. RBC mean corpuscular volume (MCV) was measured 24-weekly in all ODYSSEY participants. Samples analysed in the sub-study were collected between September 2016 and October 2020.
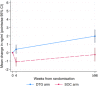
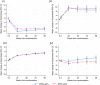
Results: A total of 229 children aged ≥6 years were included in the sub-study with median age at trial enrolment of 12.3 (interquartile range [IQR] 9.0, 14.7) years, and CD4 count of 501 (IQR 228, 695); 112 (49%) children were male. Most participants (225/229, 98%) had plasma folate results at enrolment and 214 (93%) children had results available for RBC folate, vitamin B12 and plasma folate at week ≥96. MCV results were analysed on 679 children aged ≥6 years enrolled in ODYSSEY. At week 4, mean plasma folate was significantly higher in the dolutegravir arm than in SOC (difference [DTG-SOC] 1.6 ng/ml, 95% CI 0.8, 2.3; p<0.001), and this difference persisted to week ≥96 (2.7 ng/ml, 95% CI 1.7, 3.7; p<0.001). Mean RBC folate at ≥96 weeks was also higher in the DTG arm (difference 73 ng/ml, 95% CI 3, 143; p = 0.041). There was no difference in the treatment arms for vitamin B12 levels at ≥96 weeks or change in MCV through trial follow-up.
Conclusions: Plasma and RBC folate levels were higher in children and adolescents receiving dolutegravir-based ART than on other ART regimens. Further studies are needed to clarify the mechanisms of these interactions and the clinical implications of increased blood folate levels.
Trial registration: ClinicalTrials.gov NCT02259127.
Keywords: HIV; adolescents; children; dolutegravir; folate; vitamin B12.
© 2023 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of International AIDS Society.
Conflict of interest statement
AT has received funding for serving on a ViiV Healthcare advisory board with payment made to the employer. Other authors have no conflict of interest to declare.
Figures
References
-
- Stanislaus DJ, Posobiec LM, Laffan SB, Solomon HM, Ziejewski MK, Romach EH. Absence of developmental and reproductive toxicity in animals exposed to dolutegravir. Birth Defects Res. 2020;112(3):245–261. - PubMed
-
- Smith MR, Mohan H, Ajaykumar A, Hsieh AYY, Martineau L, Patel R, et al. Second–Generation Human Immunodeficiency Virus Integrase Inhibitors Induce Differentiation Dysregulation and Exert Toxic Effects in Human Embryonic Stem Cell and Mouse Models. J Infect Dis. 2022;226(11):1992–2001. 10.1093/infdis/jiac386 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

