Bulky Substituents Promote Triplet-Triplet Annihilation Over Triplet Excimer Formation in Naphthalene Derivatives
- PMID: 37766514
- PMCID: PMC10571077
- DOI: 10.1021/jacs.3c08115
Bulky Substituents Promote Triplet-Triplet Annihilation Over Triplet Excimer Formation in Naphthalene Derivatives
Abstract
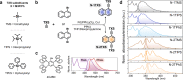
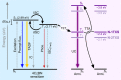
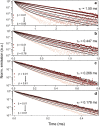
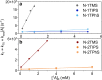
Visible-to-ultraviolet (UV) triplet-triplet annihilation photochemical upconversion (TTA-UC) has gained a lot of attention recently due to its potential for driving demanding high-energy photoreactions using low-intensity visible light. The efficiency of this process has rapidly improved in the past few years, in part thanks to the recently discovered annihilator compound 1,4-bis((triisopropylsilyl)ethynyl)naphthalene (N-2TIPS). Despite its beneficial TTA-UC characteristics, the success of N-2TIPS in this context is not yet fully understood. In this work, we seek to elucidate what role the specific type and number of substituents in naphthalene annihilator compounds play to achieve the characteristics sought after for TTA-UC. We show that the type of substituent attached to the naphthalene core is crucial for its performance as an annihilator. More specifically, we argue that the choice of substituent dictates to what degree the sensitized triplets form excimer complexes with ground state annihilators of the same type, which is a process competing with that of TTA. The addition of more bulky substituents positively impacts the upconverting ability by impeding excimer formation on the triplet surface, an effect that is enhanced with the number of substituents. The presence of triplet excimers is confirmed from transient absorption measurements, and the excimer formation rate is quantified, showing several orders of magnitude differences between different derivatives. These insights will aid in the further development of annihilator compounds for solar energy applications for which the behavior at low incident powers is of particular significance.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Enhancing Triplet-Triplet Annihilation Upconversion: From Molecular Design to Present Applications.Acc Chem Res. 2022 Sep 20;55(18):2604-2615. doi: 10.1021/acs.accounts.2c00307. Epub 2022 Sep 8. Acc Chem Res. 2022. PMID: 36074952
-
Triplet-Triplet Annihilation Upconverting Liposomes: Mechanistic Insights into the Role of Membranes in Two-Dimensional TTA-UC.ACS Appl Mater Interfaces. 2024 Jun 5;16(22):29324-29337. doi: 10.1021/acsami.4c00990. Epub 2024 May 22. ACS Appl Mater Interfaces. 2024. PMID: 38776974 Free PMC article.
-
Approaching the Spin-Statistical Limit in Visible-to-Ultraviolet Photon Upconversion.J Am Chem Soc. 2022 Mar 2;144(8):3706-3716. doi: 10.1021/jacs.1c13222. Epub 2022 Feb 17. J Am Chem Soc. 2022. PMID: 35175751 Free PMC article.
-
Recent Advances in the Photoreactions Triggered by Porphyrin-Based Triplet-Triplet Annihilation Upconversion Systems: Molecular Innovations and Nanoarchitectonics.Int J Mol Sci. 2022 Jul 21;23(14):8041. doi: 10.3390/ijms23148041. Int J Mol Sci. 2022. PMID: 35887385 Free PMC article. Review.
-
Generating spin-triplet states at the bulk perovskite/organic interface for photon upconversion.Nanoscale. 2023 Jan 19;15(3):998-1013. doi: 10.1039/d2nr05767k. Nanoscale. 2023. PMID: 36594272 Review.
Cited by
-
On the nature of the triplet electronic states of naphthalene dimers.Chem Sci. 2025 Jan 28;16(10):4469-4479. doi: 10.1039/d4sc07982e. eCollection 2025 Mar 5. Chem Sci. 2025. PMID: 39926706 Free PMC article.
-
Supramolecular Annihilator with DPA Parallelly Arranged by Multiple Hydrogen-Bonding Interactions for Enhanced Triplet-Triplet Annihilation Upconversion.Molecules. 2024 May 8;29(10):2203. doi: 10.3390/molecules29102203. Molecules. 2024. PMID: 38792064 Free PMC article.
-
Vibronic Trimer Design Enhancing Intramolecular Triplet-Exciton Hopping to Accelerate Triplet-Triplet Annihilation for Photon Upconversion.Angew Chem Int Ed Engl. 2025 Jul 21;64(30):e202503846. doi: 10.1002/anie.202503846. Epub 2025 Jun 1. Angew Chem Int Ed Engl. 2025. PMID: 40390238 Free PMC article.
References
-
- Bachilo S. M.; Weisman R. B. Determination of Triplet Quantum Yields from Triplet–Triplet Annihilation Fluorescence. J. Phys. Chem. A 2000, 104, 7713–7714. 10.1021/jp001877n. - DOI
-
- Parker C. A.; Hatchard C. G. Delayed fluorescence from solutions of anthracene and phenanthrene. Proc. R. Soc. London, Ser. A 1962, 269, 574–584. 10.1098/rspa.1962.0197. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

