Evaluation of a novel real-time polymerase chain reaction assay for identifying H3 equine influenza virus in Kazakhstan
- PMID: 37766711
- PMCID: PMC10521171
- DOI: 10.14202/vetworld.2023.1682-1689
Evaluation of a novel real-time polymerase chain reaction assay for identifying H3 equine influenza virus in Kazakhstan
Abstract
Background and aim: Equine influenza (EI) is a highly contagious disease that causes fever and upper respiratory tract inflammation. It is caused by influenza virus A, belonging to the Orthomyxoviridae family, with subtypes H3N8 and H7N7. This study presents data on the development of a real-time polymerase chain reaction (RT-PCR) assay using TaqMan probes to detect the H3 subtype of EI virus (EIV).
Materials and methods: The evaluation of the developed RT-PCR assay involved five strains of EIV as positive controls and ten nasopharyngeal swab samples collected from horses. RNA was isolated using the GeneJet Viral DNA and RNA Purification Kit, and primers and probes were designed using the Integrated DNA Technology PrimerQuest Tool. The assay was optimized by investigating the annealing temperature, primer and probes concentrations, sensitivity, and specificity. Sequencing was performed using the Thermo Fisher 3130 Genetic Analyzer, and the evolutionary history was inferred using the Neighbor-Joining method.
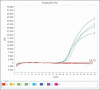
Results: The designed primers and probes, targeting the H3 gene, were found to be specific to the EIV. The RT-PCR assay was capable of detecting as low as 50 femtogram (f) or 3 × 103 copies of genomic RNA. No cross-reactions were observed with other respiratory viral and bacterial pathogens, indicating the high specificity of the assay. To evaluate its effectiveness, ten nasopharyngeal swab samples collected from farms in North Kazakhstan regions during disease monitoring were analyzed. The accuracy of the analysis was confirmed by comparing the results with those obtained from a commercial RT-PCR assay for EI identification. The developed RT-PCR assay exhibited high sensitivity and specificity for detecting the EIV.
Conclusion: The results demonstrate that the developed RT-PCR assay is suitable for diagnosing EI. This simple, highly sensitive, and specific assay for detecting H3 EIV can be a reliable tool for diagnosing and surveilling EI. Implementing this RT-PCR assay in veterinary practice will enhance and expedite the timely response to potential outbreaks of EI, thus positively impacting the overall epizootic well-being of EI in Kazakhstan.
Keywords: equine influenza; hemagglutinin; horses; primers; probe; real-time polymerase chain reaction assay; virus.
Copyright: © Sandybayev, et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Rapid detection of equine influenza virus H3N8 subtype by insulated isothermal RT-PCR (iiRT-PCR) assay using the POCKIT™ Nucleic Acid Analyzer.J Virol Methods. 2014 Oct;207:66-72. doi: 10.1016/j.jviromet.2014.06.016. Epub 2014 Jun 30. J Virol Methods. 2014. PMID: 24992669
-
Type A Influenza Virus Detection from Horses by Real-Time RT-qPCR and Insulated Isothermal RT-PCR.Methods Mol Biol. 2020;2123:383-392. doi: 10.1007/978-1-0716-0346-8_29. Methods Mol Biol. 2020. PMID: 32170704
-
Real-time RT-PCR for detection of equine influenza and evaluation using samples from horses infected with A/equine/Sydney/2007 (H3N8).Vet Microbiol. 2009 May 28;137(1-2):1-9. doi: 10.1016/j.vetmic.2008.12.006. Epub 2008 Dec 11. Vet Microbiol. 2009. PMID: 19153018
-
Equine Influenza Virus and Vaccines.Viruses. 2021 Aug 20;13(8):1657. doi: 10.3390/v13081657. Viruses. 2021. PMID: 34452521 Free PMC article. Review.
-
An Overview of Equine Influenza in South America.Viruses. 2021 May 12;13(5):888. doi: 10.3390/v13050888. Viruses. 2021. PMID: 34065839 Free PMC article. Review.
Cited by
-
Development of a Stage- and Species-Specific RNAi System for Molecular Insights in Trichogramma Wasps.Insects. 2025 Jun 27;16(7):673. doi: 10.3390/insects16070673. Insects. 2025. PMID: 40725304 Free PMC article.
References
-
- Sack A, Cullinane A, Daramragchaa U, Chuluunbaatar M, Gonchigoo B, Gray G.C. Equine influenza virus-a neglected, reemergent disease threat. Emerg. Infect. Dis. 2019;25(6):1185–1191.
-
- Dominguez M, Münstermann S, de Guindos I, Timoney P. Equine disease events resulting from international horse movements:Systematic review and lessons learned. Equine Vet. J. 2016;48(5):641–653. - PubMed
LinkOut - more resources
Full Text Sources
