Risk Factors for Cefoperazone/Sulbactam-Induced Coagulation Disorder
- PMID: 37766881
- PMCID: PMC10520255
- DOI: 10.2147/IDR.S429706
Risk Factors for Cefoperazone/Sulbactam-Induced Coagulation Disorder
Abstract
Purpose: Cefoperazone/sulbactam is a β-lactam/β-lactamase inhibitor combination effective against intra-abdominal, urinary tract, and respiratory infections. Although some studies have suggested that cefoperazone/sulbactam is associated with coagulation disorders, it remains debatable whether the combination of cefoperazone/sulbactam with tigecycline or valproic acid increases the risk of bleeding, as both drugs can lead to coagulation disorders. This study aimed to explore the risk factors of cefoperazone/sulbactam-induced coagulopathy.
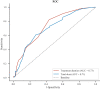
Patients and methods: This was a single-center, retrospective, nested case-control study. The sample groups were derived from individuals registered at the Department of Neurosurgery, Shanxi Provincial People's Hospital. Propensity score matching (PSM) was used to adjust for demographic data. Conditional logistic regression was used to estimate the matched odds ratios representing the odds of cefoperazone/sulbactam-induced coagulopathy (CIC), and a receiver operating characteristic curve was used to determine the optimal cut-off conditions.
Results: After PSM, 155 and 56 patients were included in the control and case groups, respectively. Multivariate analysis revealed that advanced age, treatment duration, and total dose were independent risk factors of cefoperazone/sulbactam-induced coagulation disorders. Concomitant use of vitamin K was an independent protective factor against CIC. The optimal cut-off for the length of treatment was 5 d, and the cut-off for the total dose was 48 g.
Conclusion: Tigecycline and valproic acid were not associated with CIC. Advanced age and long treatment duration are risk factors for CIC. Supplementation with vitamin K during cefoperazone/sulbactam treatment was associated with a reduced risk.
Keywords: cefoperazone/sulbactam; coagulopathy; tigecycline; valproic acid.
© 2023 Miao et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Comparison of bleeding risk and hypofibrinogenemia-associated risk factors between tigecycline with cefoperazone/sulbactam therapy and other tigecycline-based combination therapies.Front Pharmacol. 2023 Jun 7;14:1182644. doi: 10.3389/fphar.2023.1182644. eCollection 2023. Front Pharmacol. 2023. PMID: 37351509 Free PMC article.
-
A retrospective cohort study of coagulation function in patients with liver cirrhosis receiving cefoperazone/sulbactam with and without vitamin K1 supplementation.Int J Clin Pharm. 2024 Dec;46(6):1492-1499. doi: 10.1007/s11096-024-01796-w. Epub 2024 Sep 13. Int J Clin Pharm. 2024. PMID: 39269640
-
Clinical characteristics and risk factors of tigecycline-associated hypofibrinogenaemia in critically ill patients.Eur J Clin Pharmacol. 2020 Jul;76(7):913-922. doi: 10.1007/s00228-020-02860-w. Epub 2020 Apr 30. Eur J Clin Pharmacol. 2020. PMID: 32355990 Free PMC article.
-
Clinical Efficacy and Safety of Cefoperazone-Sulbactam in Treatment of Intra-Abdominal Infections: A Systematic Review and Meta-Analysis.Surg Infect (Larchmt). 2021 Oct;22(8):763-770. doi: 10.1089/sur.2020.468. Epub 2021 Feb 23. Surg Infect (Larchmt). 2021. PMID: 33625294
-
beta-Lactamase inhibition and in vitro activity of sulbactam and sulbactam/cefoperazone.Clin Infect Dis. 1997 Mar;24(3):494-7. doi: 10.1093/clinids/24.3.494. Clin Infect Dis. 1997. PMID: 9114205 Review.
Cited by
-
Prescribing Trends of Fixed-Dose Combination Antibiotics Not Recommended by the WHO (FNRs) for ICU Patients in Six Major Areas of China During a Seven-Year Period.Drug Des Devel Ther. 2024 Dec 6;18:5781-5791. doi: 10.2147/DDDT.S493980. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39664964 Free PMC article.
-
Development and internal validation of a model for predicting cefoperazone/sulbactam-associated coagulation disorders in Chinese inpatients.BMC Pharmacol Toxicol. 2024 Jul 12;25(1):41. doi: 10.1186/s40360-024-00761-7. BMC Pharmacol Toxicol. 2024. PMID: 38997770 Free PMC article.
-
Optimizing Treatment Strategies for Carbapenem-Resistant Acinetobacter Baumannii-Associated Pneumonia: A Multicenter Study in Chinese Hospitals.Infect Drug Resist. 2024 Oct 13;17:4403-4415. doi: 10.2147/IDR.S473088. eCollection 2024. Infect Drug Resist. 2024. PMID: 39421018 Free PMC article.
-
Serum trough concentration threshold and risk factors of cefoperazone-induced coagulopathy in critically ill patients: A retrospective case-control study.Eur J Clin Pharmacol. 2024 May;80(5):737-746. doi: 10.1007/s00228-024-03634-4. Epub 2024 Feb 14. Eur J Clin Pharmacol. 2024. PMID: 38353692 Free PMC article.
-
Construction and Validation of a Nomogram Prediction Model for the Risk of Cefoperazone Sodium/Sulbactam Sodium-Related Coagulation Disorders.Infect Drug Resist. 2025 Aug 1;18:3859-3866. doi: 10.2147/IDR.S534366. eCollection 2025. Infect Drug Resist. 2025. PMID: 40766047 Free PMC article.
References
-
- Greenberg RN, Reilly PM, Weinandt WJ, Bollinger M, Kennedy DJ. Cefoperazone-sulbactam combination in the treatment of urinary tract infections: efficacy, safety, and effects on coagulation. Clin Ther. 1987;10(1):52–56. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous