3D cell subculturing pillar dish for pharmacogenetic analysis and high-throughput screening
- PMID: 37766900
- PMCID: PMC10520358
- DOI: 10.1016/j.mtbio.2023.100793
3D cell subculturing pillar dish for pharmacogenetic analysis and high-throughput screening
Abstract
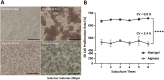
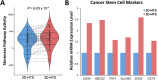
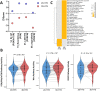
A pillar dishe for subculture of 3D cultured cells on hydrogel spots (Matrigel and alginate) have been developed. Cells cultured in 3D in an extracellular matrix (ECM) can retain their intrinsic properties, but cells cultured in 2D lose their intrinsic properties as the cells stick to the bottom of the well. Previously, cells and ECM spots were dispensed on a conventional culture dish for 3D cultivation. However, as the spot shape and location depended on user handling, pillars were added to the dish to realize uniform spot shape and stable subculture, supporting 3D cell culture-based high-throughput screening (HTS). Matrigel and alginate were used as ECMs during 6-passage subculture. The growth rate of lung cancer cell (A549) was higher on Matrigel than on alginate. Cancer cell was subcultured in three dimensions in the proposed pillar dish and used for drug screening and differential gene expression analysis. Interestingly, stemness markers, which are unique characteristics of lung cancer cells inducing drug resistance, were upregulated in 3D-subcultured cells compared with those in 2D-subcultured cells. Additionally, the PI3K/Akt/mTOR, VEGFR1/2, and Wnt pathways, which are promising therapeutic targets for lung cancer, were activated, showing high drug sensitivity under 3D-HTS using the 3D-subcultured cells.
Keywords: 3D cell culture; Cell culture dish; High-throughput screening; Micropillar and well chips.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures










Similar articles
-
High Throughput 3D Cell Migration Assay Using Micropillar/Microwell Chips.Molecules. 2022 Aug 19;27(16):5306. doi: 10.3390/molecules27165306. Molecules. 2022. PMID: 36014542 Free PMC article.
-
Cell Proliferation Receptor-Enhanced 3D High-Throughput Screening Model for Optimized Drug Efficacy Evaluation in Breast Cancer Cells.Anal Chem. 2022 Aug 30;94(34):11838-11847. doi: 10.1021/acs.analchem.2c02222. Epub 2022 Aug 17. Anal Chem. 2022. PMID: 35977405
-
An Automated High-Throughput Screening (HTS) Spotter for 3D Tumor Spheroid Formation.Int J Mol Sci. 2023 Jan 5;24(2):1006. doi: 10.3390/ijms24021006. Int J Mol Sci. 2023. PMID: 36674523 Free PMC article.
-
High-Throughput Screening of Compound Neurotoxicity Using 3D-Cultured Neural Stem Cells on a 384-Pillar Plate.Curr Protoc. 2021 Apr;1(4):e107. doi: 10.1002/cpz1.107. Curr Protoc. 2021. PMID: 33887124 Free PMC article.
-
Biocompatible Hydrogels for Microarray Cell Printing and Encapsulation.Biosensors (Basel). 2015 Oct 26;5(4):647-63. doi: 10.3390/bios5040647. Biosensors (Basel). 2015. PMID: 26516921 Free PMC article. Review.
References
-
- Gan H.K., You B., Pond G.R., Chen E.X. Assumptions of expected benefits in randomized phase III trials evaluating systemic treatments for cancer. J. Natl. Cancer Inst. 2012;104(8):590–598. - PubMed
-
- Pampaloni F., Reynaud E.G., Stelzer E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007;8(10):839–845. - PubMed
-
- Ravi M., Paramesh V., Kaviya S.R., Anuradha E., Solomon F.D. 3D cell culture systems: advantages and applications. J. Cell. Physiol. 2015;230(1):16–26. - PubMed
-
- Mayr L.M., Bojanic D. Novel trends in high-throughput screening. Curr. Opin. Pharmacol. 2009;9(5):580–588. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

