Crosstalk between integrin/FAK and Crk/Vps25 governs invasion of bovine mammary epithelial cells by S. agalactiae
- PMID: 37766995
- PMCID: PMC10520442
- DOI: 10.1016/j.isci.2023.107884
Crosstalk between integrin/FAK and Crk/Vps25 governs invasion of bovine mammary epithelial cells by S. agalactiae
Abstract
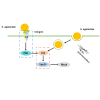
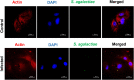
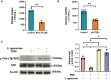
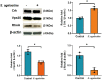
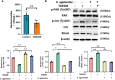
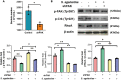
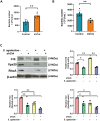
Streptococcus agalactiae (S. agalactiae) is a contagious obligate parasite of the udder in dairy cows. Here, we examined S. agalactiae-host interactions in bovine mammary epithelial cells (BMECs) in vitro. We found that S. agalactiae infected BMECs through laminin β2 and integrin. Crk, Vps25, and RhoA were differentially expressed in S. agalactiae-infected cells. S. agalactiae infection activated FAK and Crk. FAK deficiency decreased the number of intracellular S. agalactiae and Crk activation. Knockdown of Crk or Vps25 increased the level of intracellular S. agalactiae, whereas its overexpression had the opposite effect. RhoA expression and actin cytoskeleton were altered in S. agalactiae-infected BMECs. Crk and Vps25 interact in cells, and invaded S. agalactiae also activates Crk, allowing it to cooperate with Vps25 to defend against intracellular infection by S. agalactiae. This study provides insights into the mechanism by which intracellular infection by S. agalactiae is regulated in BMECs.
Keywords: Bacteriology; Cell biology; Genetics.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
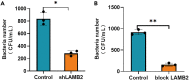
Similar articles
-
Matrine attenuates bovine mammary epithelial cells inflammatory responses induced by Streptococcus agalactiae through inhibiting NF-κB and MAPK signaling pathways.Int Immunopharmacol. 2022 Nov;112:109206. doi: 10.1016/j.intimp.2022.109206. Epub 2022 Sep 1. Int Immunopharmacol. 2022. PMID: 36058035
-
Proteomic analysis of bovine mammary epithelial cells after in vitro incubation with S. agalactiae: potential biomarkers.Vet Res. 2020 Aug 3;51(1):98. doi: 10.1186/s13567-020-00808-7. Vet Res. 2020. PMID: 32746898 Free PMC article.
-
Proteomics study on the protective mechanism of soybean isoflavone against inflammation injury of bovine mammary epithelial cells induced by Streptococcus agalactiae.Cell Stress Chaperones. 2021 Jan;26(1):91-101. doi: 10.1007/s12192-020-01158-1. Epub 2020 Aug 31. Cell Stress Chaperones. 2021. PMID: 32865767 Free PMC article.
-
Streptococcus agalactiae mastitis: a review.Can Vet J. 1997 Jul;38(7):429-37. Can Vet J. 1997. PMID: 9220132 Free PMC article. Review.
-
Update on control of Staphylococcus aureus and Streptococcus agalactiae for management of mastitis.Vet Clin North Am Food Anim Pract. 2012 Jul;28(2):203-16. doi: 10.1016/j.cvfa.2012.03.010. Epub 2012 Apr 17. Vet Clin North Am Food Anim Pract. 2012. PMID: 22664203 Review.
Cited by
-
Baicalin Attenuates Panton-Valentine Leukocidin (PVL)-Induced Cytoskeleton Rearrangement via Regulating the RhoA/ROCK/LIMK and PI3K/AKT/GSK-3β Pathways in Bovine Mammary Epithelial Cells.Int J Mol Sci. 2023 Sep 25;24(19):14520. doi: 10.3390/ijms241914520. Int J Mol Sci. 2023. PMID: 37833969 Free PMC article.
References
-
- Lin L., Huang X., Yang H., He Y., He X., Huang J., Li S., Wang X., Tang S., Liu G., Pan Z. Molecular epidemiology, antimicrobial activity, and virulence gene clustering of Streptococcus agalactiae isolated from dairy cattle with mastitis in China. J. Dairy Sci. 2021;104:4893–4903. doi: 10.3168/jds.2020-19139. - DOI - PubMed
-
- Ma F., Yang S., Wang G., Zhou M., Zhang J., Deng B., Yin W., Wang H., Lu Y., Fan H. Effect of multiplicity of infection on the evasion of neutrophil killing by Streptococcus agalactiae isolated from clinical mastitis bovine. Vet. Microbiol. 2022;270:109450. doi: 10.1016/j.vetmic.2022.109450. - DOI - PubMed
-
- Niu H., Zhang H., Wu F., Xiong B., Tong J., Jiang L. Proteomics study on the protective mechanism of soybean isoflavone against inflammation injury of bovine mammary epithelial cells induced by Streptococcus agalactiae. Cell Stress Chaperones. 2021;26:91–101. doi: 10.1007/s12192-020-01158-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

