Synthesis and application of spermine-based amphiphilic poly(β-amino ester)s for siRNA delivery
- PMID: 37767040
- PMCID: PMC10521211
- DOI: 10.1039/d3na00272a
Synthesis and application of spermine-based amphiphilic poly(β-amino ester)s for siRNA delivery
Abstract
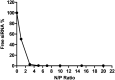
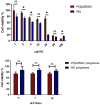
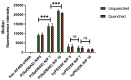
Small interfering RNA (siRNA) can trigger RNA interference (RNAi) to therapeutically silence disease-related genes in human cells. The approval of siRNA therapeutics by the FDA in recent years generated a new hope in novel and efficient siRNA therapeutics. However, their therapeutic application is still limited by the lack of safe and efficient transfection vehicles. In this study, we successfully synthesized a novel amphiphilic poly(β-amino ester) based on the polyamine spermine, hydrophobic decylamine and 1,4-butanediol diacrylate, which was characterized by 1H NMR spectroscopy and size exclusion chromatography (SEC, Mn = 6000 Da). The polymer encapsulated siRNA quantitatively from N/P 5 on as assessed by fluorescence intercalation while maintaining optimal polyplex sizes and zeta potentials. Biocompatibility and cellular delivery efficacy were also higher than those of the commonly used cationic, hyperbranched polymer polyethylenimine (PEI, 25 kDa). Optimized formulations mediated around 90% gene silencing in enhanced green fluorescence protein expressing H1299 cells (H1299-eGFP) as determined by flow cytometry. These results suggest that spermine-based, amphiphilic poly(β-amino ester)s are very promising candidates for efficient siRNA delivery.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

