Tissue factor-heparanase complex: intracellular nonhemostatic effects
- PMID: 37767062
- PMCID: PMC10520574
- DOI: 10.1016/j.rpth.2023.102179
Tissue factor-heparanase complex: intracellular nonhemostatic effects
Abstract
Background: Heparanase, known to be involved in angiogenesis, cancer progression, and inflammation, was shown to form a complex with tissue factor (TF) via its procoagulant domain and to enhance the hemostatic system.
Objectives: To reveal a potential role of heparanase procoagulant domain in nonhemostatic effects.
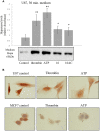
Methods: Effects of peptides 16 and 16AC derived from the heparanase procoagulant domain, discovered by our group, were studied using the XTT proliferation assay, western blot analysis, and immunostaining in vitro and a mouse wound-healing model.
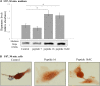
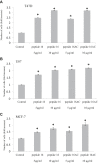
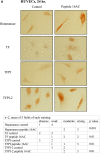
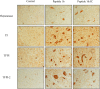
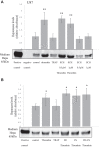
Results: Procoagulant peptides induced increased proliferation, release of heparanase, and upregulation of heparanase, TF, tissue factor pathway inhibitor (TFPI), and TFPI-2 in U87, T47D, and MCF-7 tumor cell lines and in endothelial cells. These results were reversed by a peptide derived from TFPI-2 that inhibited the heparanse procoagulant domain-TF complex. Thrombin had a similar effect on tumor cell proliferation and heparanase release, although the impact of thrombin on cell proliferation was mediated by the heparanase procoagulant domain. A mouse model of full-thickness skin incision exhibited higher levels of heparanase, TF, TFPI, and TFPI-2 in the healing skin, mainly in the blood vessel wall and lumen in animals injected with the procoagulant peptides compared to controls. The cells transfected to overexpress full-length TF or TF devoid of the cytoplasmic domain demonstrated that the procoagulant domain conveyed intracellular signaling via TF.
Conclusion: Heparanase procoagulant domain induces nonhemostatic effects via TF. The finding that TF serves as a receptor to heparanase supports the close direct relation between the hemostatic system and cancer progression.
Keywords: heparanase; neoplastic processes; receptor; tissue factor; tissue factor pathway inhibitor.
© 2023 Published by Elsevier Inc. on behalf of International Society on Thrombosis and Haemostasis.
Figures





References
-
- Pikas D.S., Li J.P., Vlodavsky I., Lindahl U. Substrate specificity of heparanases from human hepatoma and platelets. J Biol Chem. 1998;273:18770–18777. - PubMed
-
- Parish C.R., Freeman C., Hulett M.D. Heparanase: a key enzyme involved in cell invasion. Biochim Biophys Acta. 2001;1471:M99–M108. - PubMed
-
- Nadir Y., Brenner B., Zetser A., Ilan N., Shafat I., Zcharia E., Goldshmidt O., Vlodavsky I. Heparanase induces tissue factor expression in vascular endothelial and cancer cells. J Thromb Haemost. 2006;4:2443–2451. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

