Targeting phosphoglycerate kinases by tatridin A, a natural sesquiterpenoid endowed with anti-cancer activity, using a proteomic platform
- PMID: 37767160
- PMCID: PMC10519794
- DOI: 10.3389/fmolb.2023.1212541
Targeting phosphoglycerate kinases by tatridin A, a natural sesquiterpenoid endowed with anti-cancer activity, using a proteomic platform
Abstract
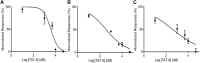
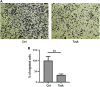
Tatridin A (TatA) is a germacrane sesquiterpenoid containing one E-double bond and one Z-double bond in its 10-membered ring, which is fused to a 3-methylene-dihydrofuran-2-one moiety. Tatridin A bioactivity has been poorly investigated despite its interesting chemical structure. Here, a functional proteomic platform was adapted to disclose its most reliable targets in leukemia monocytic cells, and phosphoglycerate kinases were recognized as the most affine enzymes. Through a combination of limited proteolysis and molecular docking, it has been discovered that tatridin A interacts with the active domains of phosphoglycerate kinase 1, altering its hinge region, and it can be accountable for tatridin A inhibition potency on enzyme activity. A more detailed tatridin A biological profile showed that it is also fully active against gastric cancer cells, downregulating the mRNA levels of chemokine receptor 4 and β-catenin and inhibiting the invasiveness of living KATO III cells as a direct consequence of phosphoglycerate kinase 1 antagonism.
Keywords: CXCR4; PGK1; cancer dissemination; functional proteomics; gastric cancer; sesquiterpenes.
Copyright © 2023 Ferraro, Voli, Mozzicafreddo, Pollastro, Tosco and Monti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

