A Yeast Cell Wall Derived Hybrid Hydrogel with Photothermal and Immune Combined Modality Therapy for Enhanced Anti-Melanoma Efficacy
- PMID: 37767196
- PMCID: PMC10520258
- DOI: 10.2147/IJN.S409674
A Yeast Cell Wall Derived Hybrid Hydrogel with Photothermal and Immune Combined Modality Therapy for Enhanced Anti-Melanoma Efficacy
Abstract
Introduction: The effect of traditional treatment for melanoma is quite limited, especially for its recurrence. As the major components of yeast cell wall, chitin and β-glucan exhibit good immune activation effect and are promising candidates for adjuvant. Therefore, melanoma cell membrane (CM) and indocyanine green (ICG) was loaded in a chitin and β-glucan hybrid hydrogel to achieve an enhanced anti-melanoma therapy.
Methods: The novel hybrid hydrogel was prepared, and its physicochemical properties were examined. Its effect towards melanoma prevention and treatment was evaluated via a melanoma-bearing mice model.
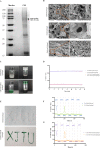
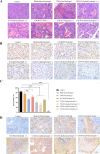
Results: The CM-ICG-hybrid hydrogel was successfully prepared with excellent injectability, self-healing, drug loading, rheological, in vitro and in vivo photothermal stability, and retention properties. It also exhibited good cellular and in vivo safety profiles. In the primary melanoma mice model, it quickly ablated the in-situ melanoma, effectively inhibited the tumor growth, increased the survival rate of melanoma-bearing mice, and increased the level of IFN-γ and TNF-α. In the distal secondary melanoma model, it efficiently prevented the reoccurrence of melanoma and activated the memory T cells. In both models, a synergistic effect of photothermal therapy and immune therapy was found. The hydrogel effectively recruited CD3+ CD4+ T cells and CD3+ CD8+ T cells, inhibited the proliferation of melanoma cells, and induced the apoptosis of melanoma cells.
Conclusion: The hybrid hydrogel was successfully prepared, and it showed excellent efficacy towards melanoma prevention and treatment due to its efficient tumor ablation and immune activation capability.
Keywords: chitin; hybrid hydrogel; immunotherapy; melanoma cell membrane; photo thermotherapy; β-glucan.
© 2023 Yang et al.
Conflict of interest statement
The authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Analgesic and potentiated photothermal therapy with ropivacaine-loaded hydrogels.Theranostics. 2023 Apr 9;13(7):2226-2240. doi: 10.7150/thno.81325. eCollection 2023. Theranostics. 2023. PMID: 37153743 Free PMC article.
-
A NIR-II light-modulated injectable self-healing hydrogel for synergistic photothermal/chemodynamic/chemo-therapy of melanoma and wound healing promotion.J Mater Chem B. 2022 Oct 5;10(38):7717-7731. doi: 10.1039/d2tb00923d. J Mater Chem B. 2022. PMID: 35920389
-
3D printed heterogeneous hybrid hydrogel scaffolds for sequential tumor photothermal-chemotherapy and wound healing.Biomater Sci. 2022 Sep 27;10(19):5648-5661. doi: 10.1039/d2bm00903j. Biomater Sci. 2022. PMID: 35994007
-
Gold nanorods-mediated efficient synergistic immunotherapy for detection and inhibition of postoperative tumor recurrence.Acta Pharm Sin B. 2021 Jul;11(7):1978-1992. doi: 10.1016/j.apsb.2021.03.035. Epub 2021 Mar 26. Acta Pharm Sin B. 2021. PMID: 34386332 Free PMC article.
-
Enhanced anti-tumor efficacy of temozolomide-loaded carboxylated poly(amido-amine) combined with photothermal/photodynamic therapy for melanoma treatment.Cancer Lett. 2018 Jun 1;423:16-26. doi: 10.1016/j.canlet.2018.03.002. Epub 2018 Mar 7. Cancer Lett. 2018. PMID: 29524557
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

