Circulating Iron in Patients with Sickle Cell Disease Mediates the Release of Neutrophil Extracellular Traps
- PMID: 37767280
- PMCID: PMC10521246
- DOI: 10.1159/000526760
Circulating Iron in Patients with Sickle Cell Disease Mediates the Release of Neutrophil Extracellular Traps
Abstract
Introduction: Neutrophils promote chronic inflammation and release neutrophil extracellular traps (NETs) that can drive inflammatory responses. Inflammation influences progression of sickle cell disease (SCD), and a role for NETs has been suggested in the onset of vaso-occlusive crisis (VOC). We aimed to identify factors in the circulation of these patients that provoke NET release, with a focus on triggers associated with hemolysis.
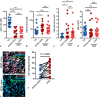
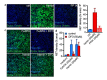
Methods: Paired serum and plasma samples during VOC and steady state of 18 SCD patients (HbSS/HbSβ0-thal and HbSC/HbSβ+-thal) were collected. Cell-free heme, hemopexin, and labile plasma iron have been measured in the plasma samples of the SCD patients. NETs formation by human neutrophils from healthy donors induced by serum of SCD patients was studied using confocal microscopy and staining for extracellular DNA using Sytox, followed by quantification of surface coverage using ImageJ.
Results: Eighteen patients paired samples obtained during VOC and steady state were available (11 HbSS/HbSβ0-thal and 7 HbSC/HbSβ+-thal). We observed high levels of systemic heme and iron, concomitant with low levels of the heme-scavenger hemopexin in sera of patients with SCD, both during VOC and in steady state. In our in vitro experiments, neutrophils released NETs when exposed to sera from SCD patients. The release of NETs was associated with high levels of circulating iron in these sera. Although hemin triggered NET formation in vitro, addition of hemopexin to scavenge heme did not suppress NET release in SCD sera. By contrast, the iron scavengers deferoxamine and apotransferrin attenuated NET formation in a significant proportion of SCD sera.
Discussion: Our results suggest that redox-active iron in the circulation of non-transfusion-dependent SCD patients activates neutrophils to release NETs, and hence, exerts a direct pro-inflammatory effect. Thus, we propose that chelation of iron requires further investigation as a therapeutic strategy in SCD.
Keywords: Hemolysis; Iron; Neutrophil extracellular traps; Neutrophils; Sickle cell disease.
Copyright © 2023 by The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


Similar articles
-
Pro-inflammatory cytokines associate with NETosis during sickle cell vaso-occlusive crises.Cytokine. 2020 Mar;127:154933. doi: 10.1016/j.cyto.2019.154933. Epub 2019 Nov 25. Cytokine. 2020. PMID: 31778959 Free PMC article.
-
Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease.Blood. 2014 Jun 12;123(24):3818-27. doi: 10.1182/blood-2013-10-529982. Epub 2014 Mar 11. Blood. 2014. PMID: 24620350 Free PMC article.
-
Dynamics of von Willebrand factor reactivity in sickle cell disease during vaso-occlusive crisis and steady state.J Thromb Haemost. 2017 Jul;15(7):1392-1402. doi: 10.1111/jth.13728. Epub 2017 Jun 5. J Thromb Haemost. 2017. PMID: 28457019
-
The Red Blood Cell-Inflammation Vicious Circle in Sickle Cell Disease.Front Immunol. 2020 Mar 13;11:454. doi: 10.3389/fimmu.2020.00454. eCollection 2020. Front Immunol. 2020. PMID: 32231672 Free PMC article. Review.
-
A novel method for high-throughput detection and quantification of neutrophil extracellular traps reveals ROS-independent NET release with immune complexes.Autoimmun Rev. 2016 Jun;15(6):577-84. doi: 10.1016/j.autrev.2016.02.018. Epub 2016 Feb 27. Autoimmun Rev. 2016. PMID: 26925759 Review.
Cited by
-
IL-8 Induces Neutrophil Extracellular Trap Formation in Severe Thermal Injury.Int J Mol Sci. 2024 Jun 29;25(13):7216. doi: 10.3390/ijms25137216. Int J Mol Sci. 2024. PMID: 39000323 Free PMC article.
-
Sickle red blood cells directly activate neutrophils.Br J Haematol. 2024 Mar;204(3):e28-e30. doi: 10.1111/bjh.19300. Epub 2024 Jan 17. Br J Haematol. 2024. PMID: 38233165 Free PMC article. No abstract available.
-
The Possible Effects of Galectin-3 on Mechanisms of Renal and Hepatocellular Injury Induced by Intravascular Hemolysis.Int J Mol Sci. 2024 Jul 25;25(15):8129. doi: 10.3390/ijms25158129. Int J Mol Sci. 2024. PMID: 39125698 Free PMC article. Review.
-
Do free radical NETwork and oxidative stress disparities in African Americans enhance their vulnerability to SARS-CoV-2 infection and COVID-19 severity?Redox Biol. 2020 Oct;37:101721. doi: 10.1016/j.redox.2020.101721. Epub 2020 Sep 15. Redox Biol. 2020. PMID: 32961440 Free PMC article. Review.
-
Severe anaemia, iron deficiency, and susceptibility to invasive bacterial infections.Wellcome Open Res. 2023 Feb 2;8:48. doi: 10.12688/wellcomeopenres.18829.1. eCollection 2023. Wellcome Open Res. 2023. PMID: 37600584 Free PMC article. Review.
References
-
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010 Dec 11;376((9757)):2018–2031. - PubMed
LinkOut - more resources
Full Text Sources

