Transfer of maternal immunity using a polyvalent vaccine and offspring protection in Nile tilapia, Oreochromis niloticus
- PMID: 37767359
- PMCID: PMC10521061
- DOI: 10.12688/f1000research.52932.4
Transfer of maternal immunity using a polyvalent vaccine and offspring protection in Nile tilapia, Oreochromis niloticus
Abstract
Background: Vaccination is an effective and alternative means of disease prevention, however, it cannot be conducted on the offspring of fish. For this process to take place, the transfer of maternal immunity should be implemented. This study aims to determine the effectiveness of transferring immunity from the broodstock to the offspring using a polyvalent vaccine against Aeromonas hydrophila, Streptococcus agalactiae, and Pseudomonas fluorescens in Nile tilapia, Oreochromis niloticus.
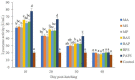
Methods: Nile tilapia broodstock with an average weight of 203g (±SD 23) was reared in spawning ponds until mass spawning and harvested one week post-spawning for vaccination. After being vaccinated according to the treatment, each fish broodstock was reared in 3x3 m cages installed in an earthen pond with a density of 20 broodstock, consisting of 15 females and 5 males. The vaccine used was a formalin-killed whole-cell vaccine at a density of 10 10 cfu/mL injected intramuscularly ( i.m.) at a dose of 0.4 mL/kg fish. Nile tilapia was injected with a vaccine used as a treatment. Example include A. hydrophila monovalent (MA) , S. agalactiae monovalent (MS) , P. fluorescens monovalent (MP), A. hydrophila and S. agalactiae bivalent (BAS) , A. hydrophila and P. fluorescens bivalent (BAP), P. fluorescens and S. agalactiae bivalent (BPS), and A. hydrophila, S. agalactiae, and P. fluorescens polyvalent vaccines (PAPS). While the control was fish that were injected with a PBS solution. The broodstock's immune response was observed on the 7 th, 14 th, 21 st, and 28 th days, while the immune response and challenge test on the offspring was conducted on the 10 th, 20 th, 30 th, and 40 th day during the post-hatching period. The parameters observed consisted of total leukocytes, phagocytic activity, antibody titer, lysozyme, and relative survival percentage (RPS).
Result: The application of PAPS in broodstock could significantly induce the best immune response and immunity to multiple diseases compared to other treatments. The RPS of the PAPS was also higher than the other types of vaccines. This showed that the transfer of immunity from the broodstock to the Nile tilapia offspring could protect it against bacterial diseases such as A. hydrophila, S. agalactiae, and P. fluorescens.
Conclusion: The application of polyvalent vaccine A. hydrophila, S. agalactiae, P. fluorescens vaccines increased the broodstock's immune response and it was transferred to their offsprings. Polyvalent vaccines derived from maternal immunity can protect offspring from disease up to 30 days of age. They were able to produce tilapia seeds that are immune to diseases caused by A. hydrophila, S. agalactiae, and P. fluorescens.
Keywords: Aeromonas hydrophila; Pseudomonas fluorescens; Streptococcus agalactiae.; bivalent vaccine; monovalent vaccine.
Copyright: © 2023 Amrullah A et al.
Conflict of interest statement
No competing interests were disclosed.
Figures



Similar articles
-
Evaluation of humoral immunity and maternal antibody transfer in Nile tilapia (Oreochromis niloticus) broodstock following immunization with a bivalent vaccine.Fish Shellfish Immunol. 2025 Oct;165:110545. doi: 10.1016/j.fsi.2025.110545. Epub 2025 Jul 5. Fish Shellfish Immunol. 2025. PMID: 40617412
-
Protection of Nile tilapia against Aeromonas hydrophila using a cobalt oxide nanoparticle vaccine containing inactivated whole cell bacteria.Dev Comp Immunol. 2025 Aug;169:105409. doi: 10.1016/j.dci.2025.105409. Epub 2025 Jul 1. Dev Comp Immunol. 2025. PMID: 40609713
-
Mucoadhesive chitosan-based nano vaccine as promising immersion vaccine against Edwardsiella tarda challenge in Nile tilapia (Oreochromis niloticus).Vet Immunol Immunopathol. 2025 Aug;286:110976. doi: 10.1016/j.vetimm.2025.110976. Epub 2025 Jul 11. Vet Immunol Immunopathol. 2025. PMID: 40663881
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Klesius P, Shoemaker C, Evans J: Streptococcus: a worldwide fish health problem. In: 8th international symposium on tilapia in aquaculture.2008;83–107.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

