Oxidative stress in rat brain during experimental status epilepticus: effect of antioxidants
- PMID: 37767398
- PMCID: PMC10520702
- DOI: 10.3389/fphar.2023.1233184
Oxidative stress in rat brain during experimental status epilepticus: effect of antioxidants
Abstract
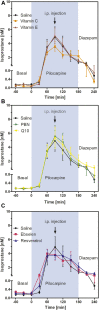
Antioxidants have been proposed as a treatment for diseases of the central nervous system. However, few studies actually studied their effects in the brain. To test central actions of antioxidants, we used the lithium-pilocarpine (Li-Pilo) model of status epilepticus (SE) in the rat in which seizures are accompanied by significant oxidative stress. We used in vivo microdialysis to determine isoprostane levels during SE in real time and brain homogenates for other measures of oxidative stress. Six different antioxidants were tested in acute and preventive experiments (vitamin C, vitamin E, ebselen, resveratrol, n-tert-butyl-α-phenylnitrone and coenzyme Q10). None of the antioxidants had an effect when given acutely during SE. In contrast, when antioxidants were given for 3 days prior to seizure induction, vitamins C and E reduced isoprostane formation by 58% and 65%, respectively. Pretreatment with the other antioxidants was ineffective. In brain homogenates prepared after 90 min of seizures, SE decreased the ratio of reduced vs. oxidized glutathione (GSH/GSSG ratio) from 60.8 to 7.50 and caused a twofold increase of 8-hydroxy-2'-deoxyguanosine (8-OHdG) levels and protein carbonyls. Pretreatment with vitamin C or vitamin E mitigated these effects and increased the GSH/GSSG ratio to 23.9 and 28.3, respectively. Again, the other antioxidants were not effective. We conclude that preventive treatment with vitamin C or vitamin E ameliorates seizure-induced oxidative damage in the brain. Several well-studied antioxidants were inactive, possibly due to limited brain permeability or a lack of chain-breaking antioxidant activity in hydrophilic compounds.
Keywords: ascorbic acid; coenzyme Q10; ebselen; isoprostanes; resveratrol; α-tocopherol.
Copyright © 2023 Fuchs, Viel, Lehto, Lau and Klein.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Ambrogini P., Albertini M. C., Betti M., Galati C., Lattanzi D., Savelli D., et al. (2018). Neurobiological correlates of alpha-tocopherol antiepileptogenic effects and microRNA expression modulation in a rat model of kainate-induced seizures. Mol. Neurobiol. 55 (10), 7822–7838. 10.1007/s12035-018-0946-7 - DOI - PMC - PubMed
-
- Betti M., Minelli A., Ambrogini P., Ciuffoli S., Viola V., Galli F., et al. (2011). Dietary ementation with α-tocopherol reduces neuroinflammation and neuronal degeneration in the rat brain after kainic acid-induced status epilepticus. Free Radic. Res. 45 (10), 1136–1142. 10.3109/10715762.2011.597750 - DOI - PubMed
LinkOut - more resources
Full Text Sources

