Study of interaction energies between residues of the active site of Hsp90 and geldanamycin analogues using quantum mechanics/molecular mechanics methods
- PMID: 37767457
- PMCID: PMC10521063
- DOI: 10.12688/f1000research.20844.2
Study of interaction energies between residues of the active site of Hsp90 and geldanamycin analogues using quantum mechanics/molecular mechanics methods
Abstract
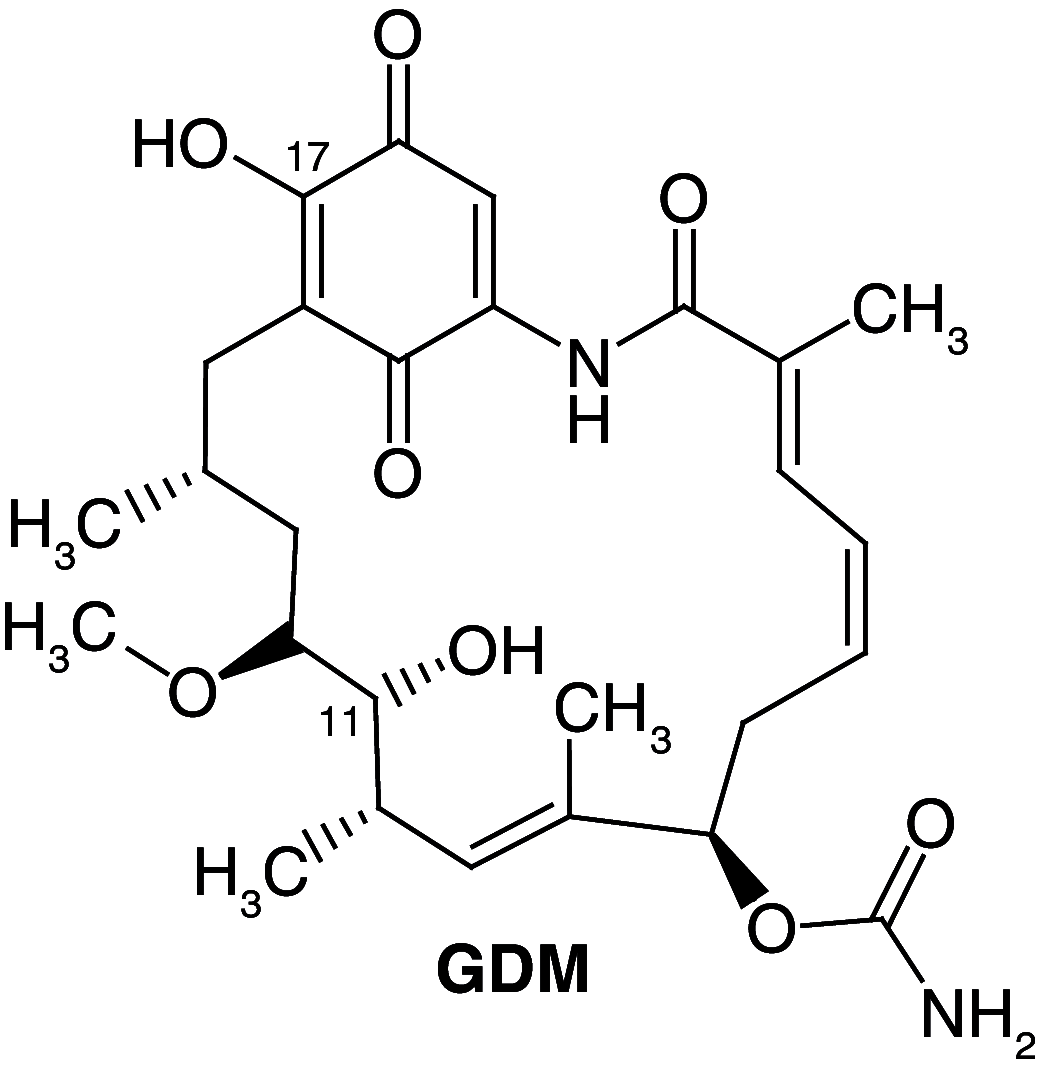
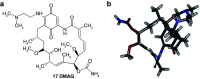
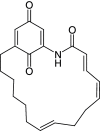
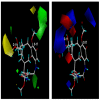
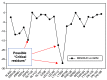
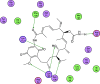
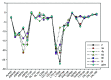
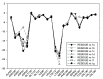
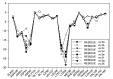
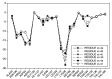
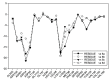
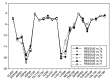
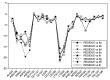
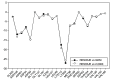
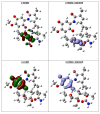
Background: Heat shock protein (Hsp90KDa) is a molecular chaperone involved in the process of cellular oncogenesis, hence its importance as a therapeutic target. Geldanamycin is an inhibitor of Hsp90 chaperone activity, which binds to the ATP binding site in the N-terminal domain of Hsp90. However, geldanamycin has shown hepatotoxic damage in clinical trials; for this reason, its use is not recommended. Taking advantage that geldanamycin binds successfully to Hsp90, many efforts have focused on the search for similar analogues, which have the same or better biological response and reduce the side effects of its predecessor; 17-AAG and 17-DMAG are examples of these analogues. Methods: In order to know the chemical factors influencing the growth or decay of the biological activity of geldanamycin analogues, different computational techniques such as docking, 3DQSAR and quantum similarity were used. Moreover, the study quantified the interaction energy between amino acids residues of active side and geldanamycin analogues, through hybrid methodology (Autodock-PM6) and DFT indexes. Results: The evaluation of interaction energies showed that the interaction with Lys58 residue is essential for the union of the analogues to the active site of Hsp90, and improves its biological activity. This union is formed through a substituent on C-11 of the geldanamycin macrocycle. A small and attractor group was found as the main steric and electrostatic characteristic that substituents on C11 need in order to interact with Lys 58; behavior was observed with hydroxy and methoxy series of geldanamycin analogues, under study. Conclusion: This study contributes with new hybrid methodology (Autodock-PM6) for the generation of 3DQSAR models, which to consider the interactions between compounds and amino acids residues of Hsp90´s active site in the alignment generation. Additionally, quantum similarity and reactivity indices calculations using DFT were performed to know the non-covalent stabilization in the active site of these compounds.
Keywords: 3D-QSAR; Hsp90; Molecular Quantum Similarity.; PM6; QM/MM approach; geldanamycin analogues.
Copyright: © 2020 Vivas-Reyes R et al.
Conflict of interest statement
No competing interests were disclosed.
Figures




Similar articles
-
Design of novel Geldanamycin analogue hsp90 alpha-inhibitor in silico for breast cancer therapy.Med Hypotheses. 2013 Sep;81(3):463-9. doi: 10.1016/j.mehy.2013.06.012. Epub 2013 Jul 13. Med Hypotheses. 2013. PMID: 23860250
-
The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation.J Biol Chem. 1997 Sep 19;272(38):23843-50. doi: 10.1074/jbc.272.38.23843. J Biol Chem. 1997. PMID: 9295332
-
Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin.Cell Stress Chaperones. 1998 Jun;3(2):100-8. doi: 10.1379/1466-1268(1998)003<0100:arbttn>2.3.co;2. Cell Stress Chaperones. 1998. PMID: 9672245 Free PMC article.
-
Hsp90 inhibitor geldanamycin and its derivatives as novel cancer chemotherapeutic agents.Curr Pharm Des. 2005;11(9):1131-8. doi: 10.2174/1381612053507585. Curr Pharm Des. 2005. PMID: 15853661 Review.
-
Geldanamycin, radicicol, and chimeric inhibitors of the Hsp90 N-terminal ATP binding site.Curr Top Med Chem. 2006;6(11):1173-82. doi: 10.2174/156802606777812031. Curr Top Med Chem. 2006. PMID: 16842154 Review.
References
-
- Workman P: Pharmacogenomics in cancer drug discovery and development: inhibitors of the Hsp90 molecular chaperone. Cancer Detect Prev. 2002;26(6):405–410. - PubMed
-
- Smith DF, Whitesell L, Katsanis E: Molecular Chaperones: Biology and Prospects for Pharmacological Intervention. Pharmacol Rev. 1998;50(4):493–513. - PubMed
Associated data
LinkOut - more resources
Full Text Sources

