Increased intrapulmonary shunt and alveolar dead space post-COVID-19
- PMID: 37767555
- PMCID: PMC10911808
- DOI: 10.1152/japplphysiol.00267.2023
Increased intrapulmonary shunt and alveolar dead space post-COVID-19
Abstract
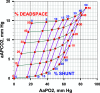
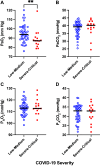
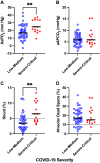
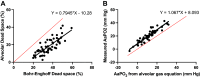
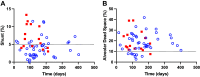
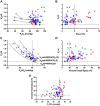
Increased intrapulmonary shunt (QS/Qt) and alveolar dead space (VD/VT) are present in early recovery from 2019 Novel Coronavirus (COVID-19). We hypothesized patients recovering from severe critical acute illness (NIH category 3-5) would have greater and longer lasting increased QS/Qt and VD/VT than patients with mild-moderate acute illness (NIH 1-2). Fifty-nine unvaccinated patients (33 males, aged 52 [38-61] yr, body mass index [BMI] 28.8 [25.3-33.6] kg/m2; median [IQR], 44 previous mild-moderate COVID-19, and 15 severe-critical disease) were studied 15-403 days postacute severe acute respiratory syndrome coronavirus infection. Breathing ambient air, steady-state mean alveolar Pco2, and Po2 were recorded simultaneously with arterial Po2/Pco2 yielding aAPco2, AaPo2, and from these, QS/Qt%, VD/VT%, and relative alveolar ventilation (40 mmHg/[Formula: see text], VArel) were calculated. Median [Formula: see text] was 39.4 [35.6-41.1] mmHg, [Formula: see text] 92.3 [87.1-98.2] mmHg; [Formula: see text] 32.8 [28.6-35.3] mmHg, [Formula: see text] 112.9 [109.4-117.0] mmHg, AaPo2 18.8 [12.6-26.8] mmHg, aAPco2 5.9 [4.3-8.0] mmHg, QS/Qt 4.3 [2.1-5.9] %, and VD/VT16.6 [12.6-24.4]%. Only 14% of patients had normal QS/Qt and VD/VT; 1% increased QS/Qt but normal VD/VT; 49% normal QS/Qt and elevated VD/VT; 36% both abnormal QS/Qt and VD/VT. Previous severe critical COVID-19 predicted increased QS/Qt (2.69 [0.82-4.57]% per category severity [95% CI], P < 0.01), but not VD/VT. Increasing age weakly predicted increased VD/VT (1.6 [0.1-3.2]% per decade, P < 0.04). Time since infection, BMI, and comorbidities were not predictors (all P > 0.11). VArel was increased in most patients. In our population, recovery from COVID-19 was associated with increased QS/Qt in 37% of patients, increased VD/VT in 86%, and increased alveolar ventilation up to ∼13 mo postinfection. NIH severity predicted QS/Qt but not elevated VD/VT. Increased VD/VT suggests pulmonary microvascular pathology persists post-COVID-19 in most patients.NEW & NOTEWORTHY Using novel methodology quantifying intrapulmonary shunt and alveolar dead space in COVID-19 patients up to 403 days after acute illness, 37% had increased intrapulmonary shunt and 86% had elevated alveolar dead space likely due to independent pathology. Elevated shunt was partially related to severe acute illness, and increased alveolar dead space was weakly related to increasing age. Ventilation was increased in the majority of patients regardless of previous disease severity. These results demonstrate persisting gas exchange abnormalities after recovery.
Keywords: alveolar dead space; hyperventilation; intrapulmonary shunt; post-COVID-19; ventilation perfusion mismatch.
Conflict of interest statement
A.M. has received consulting fees for medical education from Livanova, Eli Lilly, Zoll, and Jazz. ResMed provided a philanthropic donation to UCSD. P.D.W. has received consulting fees from SMS Biotechnology and Third pole inc. G. Prisk is an editor of
Figures


References
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727–733, 2020. doi:10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Harbut P, Prisk GK, Lindwall R, Hamzei S, Palmgren J, Farrow CE, Hedenstierna G, Amis TC, Malhotra A, Wagner PD, Kairaitis K. Intrapulmonary shunt and alveolar dead space in a cohort of patients with acute COVID-19 pneumonitis and early recovery. Eur Respir J 61: 2201117, 2023. doi:10.1183/13993003.02287-2022. - DOI - PMC - PubMed
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi:10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
-
- Tarraso J, Safont B, Carbonell-Asins JA, Fernandez-Fabrellas E, Sancho-Chust JN, Naval E, Amat B, Herrera S, Ros JA, Soler-Cataluña JJ, Rodriguez-Portal JA, Andreu AL, Marín M, Rodriguez-Hermosa JL, Gonzalez-Villaescusa C, Soriano JB, Signes-Costa J; COVID-FIBROTIC study team. Lung function and radiological findings 1 year after COVID-19: a prospective follow-up. Respir Res 23: 242, 2022. doi:10.1186/s12931-022-02166-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

