Regulatory T cells hamper the efficacy of T-cell-engaging bispecific antibody therapy
- PMID: 37767564
- PMCID: PMC10905103
- DOI: 10.3324/haematol.2023.283758
Regulatory T cells hamper the efficacy of T-cell-engaging bispecific antibody therapy
Abstract
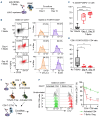
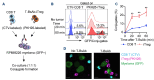
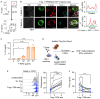
T-cell-engaging bispecific antibodies (T-BsAb) have produced impressive clinical responses in patients with relapsed/refractory B-cell malignancies, although treatment failure remains a major clinical challenge. Growing evidence suggests that a complex interplay between immune cells and tumor cells is implicated in the mechanism of action and therefore, understanding immune regulatory mechanisms might provide a clue for how to improve the efficacy of T-BsAb therapy. Here, we investigated the functional impact of regulatory T (Treg) cells on anti-tumor immunity elicited by T-BsAb therapy. In a preclinical model of myeloma, the activation and expansion of Treg cells in the bone marrow were observed in response to anti-B-cell maturation antigen (BCMA) T-BsAb therapy. T-BsAb triggered the generation of induced Treg cells from human conventional CD4 cells after co-culture with tumor cells. Moreover, T-BsAb directly activated freshly isolated circulating Treg cells, leading to the production of interleukin-10 and inhibition of T-BsAb-mediated CD8 T-cell responses. The activation of Treg cells was also seen in bone marrow samples from myeloma patients after ex vivo treatment with T-BsAb, further supporting that T-BsAb have an impact on Treg homeostasis. Importantly, transient ablation of Treg cells in combination with T-BsAb therapy dramatically improved effector lymphocyte activities and disease control in the preclinical myeloma model, leading to prolonged survival. Together, this information suggests that therapy-induced activation of Treg cells critically regulates anti-tumor immunity elicited by T-BsAb therapy, with important implications for improving the efficacy of such treatment.
Figures



References
-
- Bannerji R, Arnason JE, Advani RH, et al. . Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022;9(5):e327-e339. - PMC - PubMed
-
- Chari A, Minnema MC, Berdeja JG, et al. . Talquetamab, a T-cell-redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med. 2022;387(24):2232-2244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

