Expression of human thrombomodulin by GalTKO.hCD46 pigs modulates coagulation cascade activation by endothelial cells and during ex vivo lung perfusion with human blood
- PMID: 37767640
- PMCID: PMC10840969
- DOI: 10.1111/xen.12828
Expression of human thrombomodulin by GalTKO.hCD46 pigs modulates coagulation cascade activation by endothelial cells and during ex vivo lung perfusion with human blood
Abstract
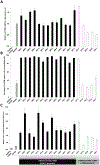
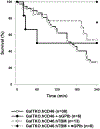
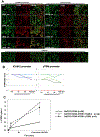
Thrombomodulin is important for the production of activated protein C (APC), a molecule with significant regulatory roles in coagulation and inflammation. To address known molecular incompatibilities between pig thrombomodulin and human thrombin that affect the conversion of protein C into APC, GalTKO.hCD46 pigs have been genetically modified to express human thrombomodulin (hTBM). The aim of this study was to evaluate the impact of transgenic hTBM expression on the coagulation dysregulation that is observed in association with lung xenograft injury in an established lung perfusion model, with and without additional blockade of nonphysiologic interactions between pig vWF and human GPIb axis. Expression of hTBM was variable between pigs at the transcriptional and protein level. hTBM increased the activation of human protein C and inhibited thrombosis in an in vitro flow perfusion assay, confirming that the expressed protein was functional. Decreased platelet activation was observed during ex vivo perfusion of GalTKO.hCD46 lungs expressing hTBM and, in conjunction with transgenic hTBM, blockade of the platelet GPIb receptor further inhibited platelets and increased survival time. Altogether, our data indicate that expression of transgenic hTBM partially addresses coagulation pathway dysregulation associated with pig lung xenograft injury and, in combination with vWF-GP1b-directed strategies, is a promising approach to improve the outcomes of lung xenotransplantation.
Keywords: activated protein C; coagulation; ex vivo perfusion; lung; thrombomodulin; xenotransplantation.
© 2023 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

