Gold-Catalyzed Cyclization of Yndiamides with Isoxazoles via α-Imino Gold Fischer Carbenes
- PMID: 37767940
- PMCID: PMC10947298
- DOI: 10.1002/chem.202302821
Gold-Catalyzed Cyclization of Yndiamides with Isoxazoles via α-Imino Gold Fischer Carbenes
Abstract
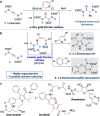
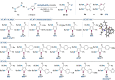
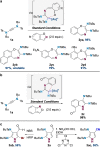
Gold catalysis is an important method for alkyne functionalization. Here we report the gold-catalyzed formal [3+2] aminative cyclization of yndiamides and isoxazoles in a direct synthesis of polysubstituted diaminopyrroles, which are important motifs in drug discovery. Key to this process is the formation, and subsequent cyclization, of an α-imino gold Fischer carbene, which represents a new type of gold carbene intermediate. The reaction proceeds rapidly under mild conditions, with high regioselectivity being achieved by introducing a subtle steric bias between the nitrogen substituents on the yndiamide. DFT calculations revealed that the key to this regioselectivity was the interconversion of isomeric gold keteniminiun ions via a low-barrier π-complex transition state, which establishes a Curtin-Hammett scenario for isoxazole addition. By using benzisoxazoles as substrates, the reaction outcome could be switched to a formal [5+2] cyclization, leading to 1,4-oxazepines.
Keywords: carbene; cyclization; gold catalysis; pyrrole; yndiamide.
© 2023 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Au(I)-Catalyzed Oxidative Functionalization of Yndiamides.Org Lett. 2021 Jun 18;23(12):4888-4892. doi: 10.1021/acs.orglett.1c01625. Epub 2021 Jun 3. Org Lett. 2021. PMID: 34080872
-
Transition Metal-Catalyzed Tandem Reactions of Ynamides for Divergent N-Heterocycle Synthesis.Acc Chem Res. 2020 Sep 15;53(9):2003-2019. doi: 10.1021/acs.accounts.0c00417. Epub 2020 Sep 1. Acc Chem Res. 2020. PMID: 32869969
-
DFT Rationalization of Gold(I)-Catalyzed Couplings between Alkynyl Thioether and Nitrenoid Derivatives: Mechanism, Selectivity Patterns, and Effects of Substituents.J Org Chem. 2022 Jun 3;87(11):7193-7201. doi: 10.1021/acs.joc.2c00407. Epub 2022 May 17. J Org Chem. 2022. PMID: 35579210
-
Computation-guided development of Au-catalyzed cycloisomerizations proceeding via 1,2-Si or 1,2-H migrations: regiodivergent synthesis of silylfurans.J Am Chem Soc. 2010 Jun 9;132(22):7645-55. doi: 10.1021/ja910290c. J Am Chem Soc. 2010. PMID: 20476771 Free PMC article.
-
Gold-Catalysed Nitroalkyne Cycloisomerization - Synthetic Utility.Chem Rec. 2021 Dec;21(12):3546-3558. doi: 10.1002/tcr.202100111. Epub 2021 Jun 1. Chem Rec. 2021. PMID: 34075681 Review.
Cited by
-
Gold-Catalyzed Diyne-Ene Annulation for the Synthesis of Polysubstituted Benzenes through Formal [3+3] Approach with Amide as the Critical Co-Catalyst.Angew Chem Int Ed Engl. 2024 Sep 23;63(39):e202407360. doi: 10.1002/anie.202407360. Epub 2024 Aug 21. Angew Chem Int Ed Engl. 2024. PMID: 38973064 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

