Gold-Catalyzed Cyclization of Yndiamides with Isoxazoles via α-Imino Gold Fischer Carbenes
- PMID: 37767940
- PMCID: PMC10947298
- DOI: 10.1002/chem.202302821
Gold-Catalyzed Cyclization of Yndiamides with Isoxazoles via α-Imino Gold Fischer Carbenes
Abstract
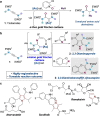
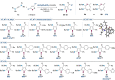
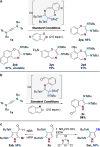
Gold catalysis is an important method for alkyne functionalization. Here we report the gold-catalyzed formal [3+2] aminative cyclization of yndiamides and isoxazoles in a direct synthesis of polysubstituted diaminopyrroles, which are important motifs in drug discovery. Key to this process is the formation, and subsequent cyclization, of an α-imino gold Fischer carbene, which represents a new type of gold carbene intermediate. The reaction proceeds rapidly under mild conditions, with high regioselectivity being achieved by introducing a subtle steric bias between the nitrogen substituents on the yndiamide. DFT calculations revealed that the key to this regioselectivity was the interconversion of isomeric gold keteniminiun ions via a low-barrier π-complex transition state, which establishes a Curtin-Hammett scenario for isoxazole addition. By using benzisoxazoles as substrates, the reaction outcome could be switched to a formal [5+2] cyclization, leading to 1,4-oxazepines.
Keywords: carbene; cyclization; gold catalysis; pyrrole; yndiamide.
© 2023 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

