The Spectrum of HPV-independent Penile Intraepithelial Neoplasia: A Proposal for Subclassification
- PMID: 37768009
- PMCID: PMC10642695
- DOI: 10.1097/PAS.0000000000002130
The Spectrum of HPV-independent Penile Intraepithelial Neoplasia: A Proposal for Subclassification
Abstract
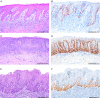
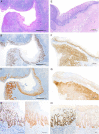
Compared with vulva, precursor lesions of human papillomavirus (HPV)-independent invasive squamous cell carcinoma (SCC) of the penis are insufficiently characterized. We analyzed the histologic and immunohistochemical characteristics of 70 peritumoral precursor lesions and correlated them with the histology and mutational profile of the adjacent HPV-negative invasive penile SCC. Atypical basal keratinocyte proliferation with variously elongated epithelial rete with premature squamatiziation, but regular superficial cornification, termed differentiated penile intraepithelial neoplasia (d-PeIN), were identified adjacent to 42/70 (60%) SCC (36/42 keratinizing ( P <0.001); 3 papillary, and 1 each verrucous, clear cell, sarcomatoid SCC). d-PeIN were associated with chronic inflammatory dermatoses (32/42; P <0.001), p53 overexpression (26/42; P <0.001), and hotspot mutations in TP53 (32/42; P <0.001), CDKN2A (26/42; P <0.001) or both (21/42; P =0.003) in the adjacent SCC. Cytoplasmic p16 ink4a overexpression in 5/42 d-PeIN correlated with CDKN2A missense mutations in the adjacent SCC. In all, 21/70 (30%) cornified verrucous or glycogenated verruciform precursors with minimal atypia and wild-type p53 (18/21; P <0.001) occurred adjacent to verrucous or papillary SCC (17/21; P <0.001) and keratinizing (4/21) SCC, which harbored mutations in HRAS and/or PIK3CA (12/21; P <0.004). Undifferentiated p16 ink4a -negative full-thickness precursors were identified in 7/70 (10%) SCC. Four histologically different HPV-independent penile precursor lesions can be assigned to 2 major genetic/biological pathways with characteristic highly differentiated precursors requiring different clinical management decisions. These include d-PeIN in chronic inflammatory dermatoses, with p53 overexpression and TP53/CDKN2A mutations, and the p53 wild-type verrucous and verruciform precursors unassociated with dermatoses, but with mutations in oncogenes PIK3CA and HRAS .
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Figures



Similar articles
-
The Histologic and Molecular Spectrum of Highly Differentiated HPV-independent Cervical Intraepithelial Neoplasia.Am J Surg Pathol. 2023 Aug 1;47(8):942-949. doi: 10.1097/PAS.0000000000002067. Epub 2023 Jun 7. Am J Surg Pathol. 2023. PMID: 37283469
-
HPV-independent, p53-wild-type vulvar intraepithelial neoplasia: a review of nomenclature and the journey to characterize verruciform and acanthotic precursor lesions of the vulva.Mod Pathol. 2022 Oct;35(10):1317-1326. doi: 10.1038/s41379-022-01079-7. Epub 2022 Apr 18. Mod Pathol. 2022. PMID: 35437330 Review.
-
TP53 Wild-Type, Human Papillomavirus-Independent Anal Growth/(Intra)Epithelial Lesion (ANGEL): A Potential Precursor of Anal Squamous Cell Carcinoma.Mod Pathol. 2025 May;38(5):100721. doi: 10.1016/j.modpat.2025.100721. Epub 2025 Jan 23. Mod Pathol. 2025. PMID: 39863109
-
Molecular characterization of invasive and in situ squamous neoplasia of the vulva and implications for morphologic diagnosis and outcome.Mod Pathol. 2021 Feb;34(2):508-518. doi: 10.1038/s41379-020-00651-3. Epub 2020 Aug 13. Mod Pathol. 2021. PMID: 32792599
-
WHO 2022 classification of penile and scrotal cancers: updates and evolution.Histopathology. 2023 Mar;82(4):508-520. doi: 10.1111/his.14824. Epub 2022 Nov 9. Histopathology. 2023. PMID: 36221864 Review.
Cited by
-
Simultaneous p53 and p16 Immunostaining for Molecular Subclassification of Head and Neck Squamous Cell Carcinomas.Head Neck Pathol. 2024 Aug 7;18(1):73. doi: 10.1007/s12105-024-01680-z. Head Neck Pathol. 2024. PMID: 39110300 Free PMC article.
-
Human Papillomavirus-Associated Giant Clear Cell Acanthoma and Squamous Cell Carcinoma: A Rare Case Report and Literature Review.J Clin Med. 2024 Apr 24;13(9):2482. doi: 10.3390/jcm13092482. J Clin Med. 2024. PMID: 38731009 Free PMC article. Review.
References
-
- Alvarado-Cabrero I, Chaux A, Muneer A, et al. . HPV-associated squamous cell carcinoma WHO Classification of Tumours Editorial Board. WHO Classification of Tumors: Urinary and Male Genital Tumours. 2022IARC. 372–374.
-
- Alvarado-Cabrero I, Canete-Portillo S, Chaux A, et al. . HPV-independent squamous cell carcinoma WHO Classification of Tumours Editorial Board. WHO Classification of Tumours. 2022International Agency for Research on Cancer. 375–379.
-
- Miralles-Guri C, Bruni L, Cubilla AL, et al. . Human papillomavirus prevalence and type distribution in penile carcinoma. J Clin Pathol. 2009;62:870–878. - PubMed
-
- Sand FL, Rasmussen CL, Frederiksen MH, et al. . Prognostic significance of HPV and p16 status in men diagnosed with penile cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2018;27:1123–1132. - PubMed
-
- Straub Hogan MM, Spieker AJ, Orejudos M, et al. . Pathological characterization and clinical outcome of penile intraepithelial neoplasia variants: a North American series. Mod Pathol. 2022;35:1101–1109. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

