Systemic application of bone-targeting peptidoglycan hydrolases as a novel treatment approach for staphylococcal bone infection
- PMID: 37768041
- PMCID: PMC10653945
- DOI: 10.1128/mbio.01830-23
Systemic application of bone-targeting peptidoglycan hydrolases as a novel treatment approach for staphylococcal bone infection
Abstract
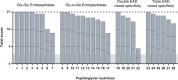
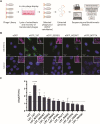
The rising prevalence of antimicrobial resistance in S. aureus has rendered treatment of staphylococcal infections increasingly difficult, making the discovery of alternative treatment options a high priority. Peptidoglycan hydrolases, a diverse group of bacteriolytic enzymes, show high promise as such alternatives due to their rapid and specific lysis of bacterial cells, independent of antibiotic resistance profiles. However, using these enzymes for the systemic treatment of local infections, such as osteomyelitis foci, needs improvement, as the therapeutic distributes throughout the whole host, resulting in low concentrations at the actual infection site. In addition, the occurrence of intracellularly persisting bacteria can lead to relapsing infections. Here, we describe an approach using tissue-targeting to increase the local concentration of therapeutic enzymes in the infected bone. The enzymes were modified with a short targeting moiety that mediated accumulation of the therapeutic in osteoblasts and additionally enables targeting of intracellularly surviving bacteria.
Keywords: MRSA; Staphylococcus aureus; antibiotic resistance; bacteriophages; cell-penetrating homing peptide; endolysin; osteomyelitis; peptidoglycan hydrolase; phage display; protein therapeutics; tissue-targeting.
Conflict of interest statement
M.J.L. is a scientific advisor for Micreos, a company producing bacteriophage-based antimicrobials. A.P.K., M.H., C.R., F.E., and M.S. are currently employees of Micreos.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

