Carboxylate Catalysis: A Catalytic O-Silylative Aldol Reaction of Aldehydes and Ethyl Diazoacetate
- PMID: 37768196
- PMCID: PMC10594658
- DOI: 10.1021/acs.joc.3c01304
Carboxylate Catalysis: A Catalytic O-Silylative Aldol Reaction of Aldehydes and Ethyl Diazoacetate
Abstract
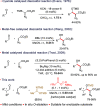
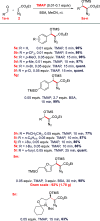
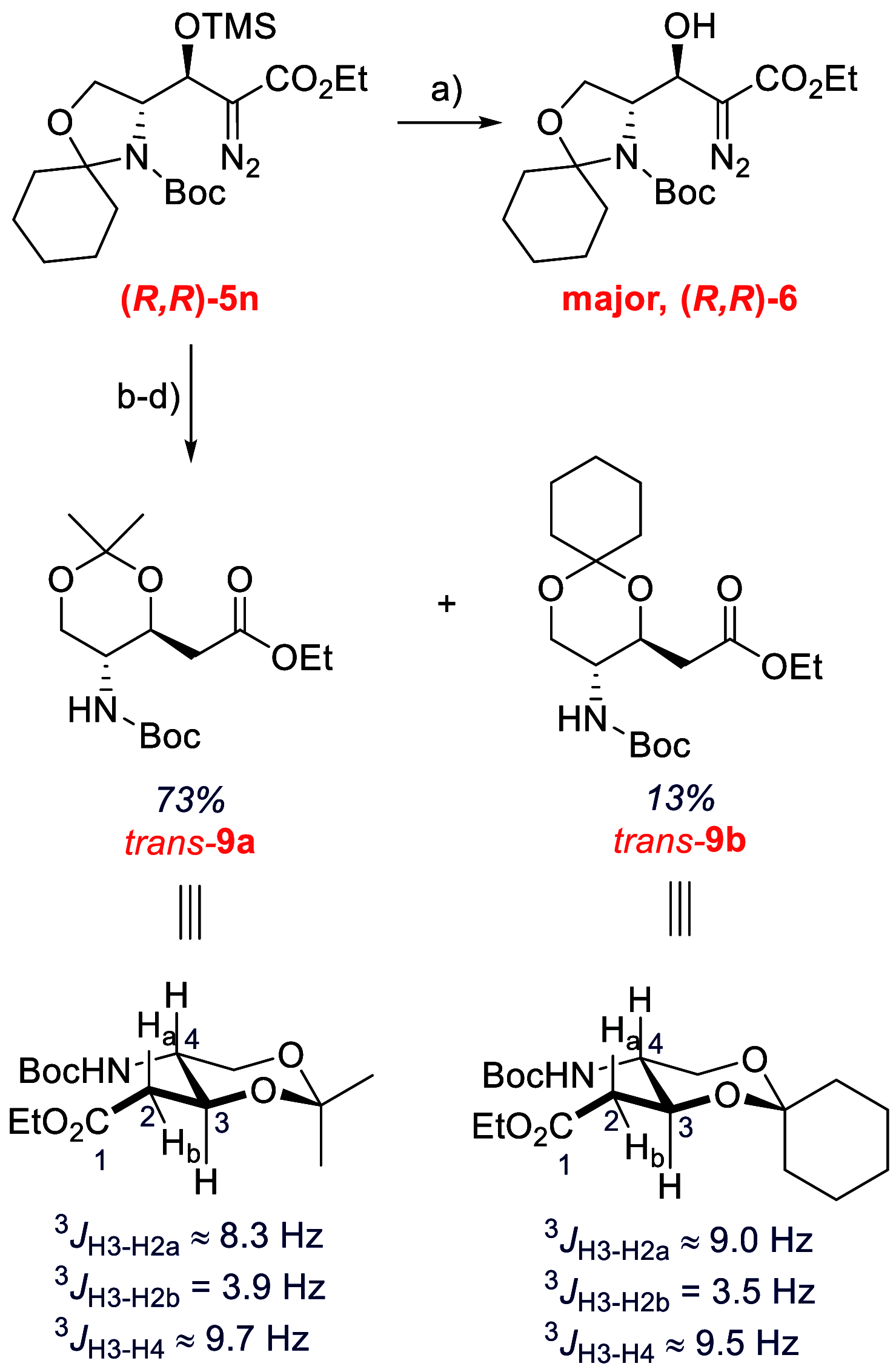
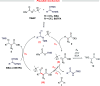
A mild catalytic variant of the aldol reaction between ethyl diazoacetate and aldehydes is described using a combination of N,O-bis(trimethylsilyl)acetamide and catalytic tetramethylammonium pivalate as catalyst. The reaction proceeds rapidly at ambient temperature to afford the O-silylated aldol products in good to excellent yield, and the acetamide byproducts can be removed by simple filtration.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Direct catalytic asymmetric aldol-type reaction of aldehydes with ethyl diazoacetate.Org Lett. 2003 May 1;5(9):1527-30. doi: 10.1021/ol0343257. Org Lett. 2003. PMID: 12713315
-
N-heterocyclic carbene-catalyzed Mukaiyama aldol reactions.Org Lett. 2007 Mar 15;9(6):1013-6. doi: 10.1021/ol0630494. Epub 2007 Feb 13. Org Lett. 2007. PMID: 17295498
-
Diarylprolinol as an Effective Organocatalyst in Asymmetric Cross-Aldol Reactions of Two Different Aldehydes.Chem Rec. 2023 Jul;23(7):e202200159. doi: 10.1002/tcr.202200159. Epub 2022 Jul 27. Chem Rec. 2023. PMID: 35896950 Review.
-
Evans-Tishchenko coupling of heteroaryl aldehydes.J Org Chem. 2010 Nov 5;75(21):7475-8. doi: 10.1021/jo1015689. J Org Chem. 2010. PMID: 20929205
-
[Development of highly stereoselective reactions utilizing heteroatoms--asymmetric synthesis of alpha-substituted serines].Yakugaku Zasshi. 2000 Jan;120(1):28-41. doi: 10.1248/yakushi1947.120.1_28. Yakugaku Zasshi. 2000. PMID: 10655780 Review. Japanese.
Cited by
-
Carboxylate-Catalyzed C-Silylation of Terminal Alkynes.Org Lett. 2024 Mar 15;26(10):1991-1995. doi: 10.1021/acs.orglett.3c04213. Epub 2024 Mar 1. Org Lett. 2024. PMID: 38428925 Free PMC article.
References
-
- Mukaiyama T.The Directed Aldol Reaction. In Organic Reactions; Dauben W. G.et al. Eds.; Wiley, 2005; Vol. 28, pp 203–331. DOI: 10.1002/0471264180.or028.03; . - DOI
- Guthrie J. P. Equilibrium constants for a series of simple aldol condensations, and linear free energy relations with other carbonyl addition reactions. Can. J. Chem. 1978, 56 (7), 962–973. 10.1139/v78-162. - DOI
- Guthrie J. P.; Wang X.-P. The aldol condensation of acetophenone with acetone. Can. J. Chem. 1991, 69 (2), 339–344. 10.1139/v91-052. - DOI
-
- Evans D. A.; Tedrow J. S.; Shaw J. T.; Downey C. W. Diastereoselective Magnesium Halide-Catalyzed anti-Aldol Reactions of Chiral N-Acyloxazolidinones. J. Am. Chem. Soc. 2002, 124 (3), 392–393. 10.1021/ja0119548. - DOI - PubMed
- Chini M.; Crotti P.; Gardelli C.; Minutolo F.; Pineschi M. Metal Salt-Promoted Aldol Reaction of Silyl Enol Ethers with Aldehydes. Gazz. Chim. Ital. 1993, 123, 673–676.
- Kiyooka S.-I.; Tsutsui T.; Maeda H.; Kaneko Y.; Isobe K. A Novel Nitroaldol Reaction Catalyzed by Rhodium Complex in the Presence of a Silyl Ketene Acetal. Tetrahedron Lett. 1995, 36, 6531–6534. 10.1016/0040-4039(95)01314-8. - DOI
- Li C.-T.; Liu H.; Xu Y.-J.; Lu C.-D. Aldol Reaction of N-tert-Butanesulfinyl Imidates under Basic Conditions for Diastereoselective Synthesis of anti-Aldols. J. Org. Chem. 2017, 82 (20), 11253–11261. 10.1021/acs.joc.7b01982. - DOI - PubMed
- Huang G.; Shrestha R.; Jia K.; Geisbrecht B. V.; Li P. Enantioselective Synthesis of Dilignol Model Compounds and Their Stereodiscrimination Study with a Dye-Decolorizing Peroxidase. Org. Lett. 2017, 19 (7), 1820–1823. 10.1021/acs.orglett.7b00587. - DOI - PMC - PubMed
- Evans D. A.; Downey C. W.; Shaw J. T.; Tedrow J. S. Magnesium Halide-Catalyzed Anti-Aldol Reactions of Chiral N-Acylthiazolidinethiones. Org. Lett. 2002, 4 (7), 1127–1130. 10.1021/ol025553o. - DOI - PubMed
-
- Heaney H.; Cui J.. N,O-Bis(trimethylsilyl)acetamide. In Encyclopedia of Reagents for Organic Synthesis; Wiley, 2007. 10.1002/047084289X.rb208.pub2. - DOI
-
- Wenkert E.; McPherson C. A. Condensations of Acyldiazomethanes with Aldehydes, Ketones, and Their Derivatives. J. Am. Chem. Soc. 1972, 94 (23), 8084–8090. 10.1021/ja00778a025. - DOI
-
- Evans D. A.; Truesdale L. K.; Grimm K. G. Carbonyl Insertion Reactions of Ethyl α-Trimethylsilyldiazoacetate. An Improved Route to Diazoacetate Aldol Products. J. Org. Chem. 1976, 41 (20), 3335–3336. 10.1021/jo00882a034. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

