Oral Versus Subcutaneous Methotrexate in Immune-Mediated Inflammatory Disorders: an Update of the Current Literature
- PMID: 37768405
- PMCID: PMC10754736
- DOI: 10.1007/s11926-023-01116-7
Oral Versus Subcutaneous Methotrexate in Immune-Mediated Inflammatory Disorders: an Update of the Current Literature
Abstract
Purpose: This review aims to critically evaluate the potential benefit of either oral or subcutaneous administration of methotrexate (MTX) in various immune-mediated inflammatory disorders (IMIDs) through analysis of efficacy, toxicity, pharmacokinetics and pharmacodynamics of both administration routes.
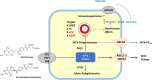
Recent findings: Recent studies comparing the efficacy of oral versus subcutaneous MTX administration in IMIDs have revealed contradicting results. Some reported higher efficacy with subcutaneous administration, while others found no significant difference. Regarding toxicity, some studies have challenged the notion that subcutaneous administration is better tolerated than oral administration, while others have supported this. Pharmacokinetic studies suggest higher plasma bioavailability and increased accumulation of MTX-polyglutamates (MTX-PGs) in red blood cells (RBCs) with subcutaneous administration during the initial treatment phase. However, after several months, similar intracellular drug levels are observed with both administration routes. There is no conclusive evidence supporting the superiority of either oral or subcutaneous MTX administration in terms of efficacy and adverse events in IMIDs. Subcutaneous administration leads to higher plasma bioavailability and initial accumulation of MTX-PGs in RBCs, but the difference seems to disappear over time. Given the variable findings, the choice of administration route may be based on shared decision-making, offering patients the option of either oral or subcutaneous administration of MTX based on individual preferences and tolerability. Further research is needed to better understand the impact of MTX-PGs in various blood cells and TDM on treatment response and adherence to MTX therapy.
Keywords: Crohn’s disease; Inflammatory bowel disease; Juvenile idiopathic arthritis; Methotrexate; Methotrexate polyglutamates; Rheumatoid arthritis.
© 2023. The Author(s).
Conflict of interest statement
All authors have complete the ICMJE disclosure form declaring whether there are conflicts of interest with this article.
Figures
Similar articles
-
A meta-analysis of methotrexate polyglutamates in relation to efficacy and toxicity of methotrexate in inflammatory arthritis, colitis and dermatitis.Br J Clin Pharmacol. 2023 Jan;89(1):61-79. doi: 10.1111/bcp.15579. Epub 2022 Nov 20. Br J Clin Pharmacol. 2023. PMID: 36326810 Free PMC article.
-
Methotrexate in rheumatoid arthritis: optimizing therapy among different formulations. Current and emerging paradigms.Clin Ther. 2014 Mar 1;36(3):427-35. doi: 10.1016/j.clinthera.2014.01.014. Epub 2014 Mar 5. Clin Ther. 2014. PMID: 24612941 Review.
-
Methotrexate and Rheumatoid Arthritis: Current Evidence Regarding Subcutaneous Versus Oral Routes of Administration.Adv Ther. 2016 Mar;33(3):369-78. doi: 10.1007/s12325-016-0295-8. Epub 2016 Feb 4. Adv Ther. 2016. PMID: 26846283 Free PMC article. Review.
-
Pharmacokinetics of oral and subcutaneous methotrexate in red and white blood cells in patients with early rheumatoid arthritis: the methotrexate monitoring trial.Ann Rheum Dis. 2023 Apr;82(4):460-467. doi: 10.1136/ard-2022-223398. Epub 2022 Dec 21. Ann Rheum Dis. 2023. PMID: 36543526 Clinical Trial.
-
Methotrexate bioavailability after oral and subcutaneous dministration in children with juvenile idiopathic arthritis.Clin Exp Rheumatol. 2009 Nov-Dec;27(6):1047-53. Clin Exp Rheumatol. 2009. PMID: 20149329
Cited by
-
Systematic review: genetic polymorphisms in the pharmacokinetics of high-dose methotrexate in pediatric acute lymphoblastic leukemia patients.Cancer Chemother Pharmacol. 2024 Aug;94(2):141-155. doi: 10.1007/s00280-024-04694-0. Epub 2024 Jul 13. Cancer Chemother Pharmacol. 2024. PMID: 39002021
-
Region-specific, data-driven guidelines are needed for rheumatic diseases in LMICs.Nat Rev Rheumatol. 2025 Aug;21(8):445-446. doi: 10.1038/s41584-025-01265-2. Nat Rev Rheumatol. 2025. PMID: 40355580 No abstract available.
References
-
- Jones G, Crotty M, Brooks P. Interventions for psoriatic arthritis. Cochrane Database Syst Rev. 2000(3):Cd000212. 10.1002/14651858.Cd000212. - PubMed
-
- Smolen JS, Landewé RBM, Bergstra SA, Kerschbaumer A, Sepriano A, Aletaha D, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82(1):3–18. doi: 10.1136/ard-2022-223356. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

