Recombinant Erythropoietin Induces Oligodendrocyte Progenitor Cell Proliferation After Traumatic Brain Injury and Delayed Hypoxemia
- PMID: 37768487
- PMCID: PMC10684442
- DOI: 10.1007/s13311-023-01443-8
Recombinant Erythropoietin Induces Oligodendrocyte Progenitor Cell Proliferation After Traumatic Brain Injury and Delayed Hypoxemia
Abstract
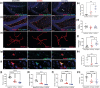
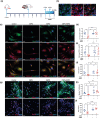
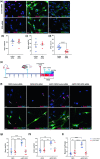
Traumatic brain injury (TBI) can result in axonal loss and demyelination, leading to persistent damage in the white matter. Demyelinated axons are vulnerable to pathologies related to an abnormal myelin structure that expose neurons to further damage. Oligodendrocyte progenitor cells (OPCs) mediate remyelination after recruitment to the injury site. Often this process is inefficient due to inadequate OPC proliferation. To date, no effective treatments are currently available to stimulate OPC proliferation in TBI. Recombinant human erythropoietin (rhEPO) is a pleiotropic neuroprotective cytokine, and its receptor is present in all stages of oligodendroglial lineage cell differentiation. Therefore, we hypothesized that rhEPO administration would enhance remyelination after TBI through the modulation of OPC response. Utilizing a murine model of controlled cortical impact and a primary OPC culture in vitro model, we characterized the impact of rhEPO on remyelination and proliferation of oligodendrocyte lineage cells. Myelin black gold II staining of the peri-contusional corpus callosum revealed an increase in myelinated area in association with an increase in BrdU-positive oligodendrocytes in injured mice treated with rhEPO. Furthermore, morphological analysis of OPCs showed a decrease in process length in rhEPO-treated animals. RhEPO treatment increased OPC proliferation after in vitro CSPG exposure. Erythropoietin receptor (EPOr) gene knockdown using siRNA prevented rhEPO-induced OPC proliferation, demonstrating that the rhEPO effect on OPC response is EPOr activation dependent. Together, our findings demonstrate that rhEPO administration may promote myelination by increasing oligodendrocyte lineage cell proliferation after TBI.
Keywords: Erythropoietin; Myelination; Oligodendrocyte progenitor cells; Traumatic brain injury; White matter injury.
© 2023. The American Society for Experimental Neurotherapeutics, Inc.
Conflict of interest statement
The authors have no conflict of interests.
Figures



Similar articles
-
Oligodendrocyte progenitor cells' fate after neonatal asphyxia-Puzzling implications for the development of hypoxic-ischemic encephalopathy.Brain Pathol. 2024 Nov;34(6):e13255. doi: 10.1111/bpa.13255. Epub 2024 Mar 19. Brain Pathol. 2024. PMID: 38504469 Free PMC article.
-
C1ql1 expression in oligodendrocyte progenitor cells promotes oligodendrocyte differentiation.FEBS J. 2025 Jan;292(1):52-74. doi: 10.1111/febs.17256. Epub 2024 Sep 11. FEBS J. 2025. PMID: 39257292 Free PMC article.
-
Grafted human-induced pluripotent stem cells-derived oligodendrocyte progenitor cells combined with human umbilical vein endothelial cells contribute to functional recovery following spinal cord injury.Stem Cell Res Ther. 2024 Feb 7;15(1):35. doi: 10.1186/s13287-024-03651-1. Stem Cell Res Ther. 2024. PMID: 38321505 Free PMC article.
-
A New Acquaintance of Oligodendrocyte Precursor Cells in the Central Nervous System.Neurosci Bull. 2024 Oct;40(10):1573-1589. doi: 10.1007/s12264-024-01261-8. Epub 2024 Jul 23. Neurosci Bull. 2024. PMID: 39042298 Review.
-
Neurorehabilitation and white matter repair in traumatic spinal cord injury: a dialogue between clinical and preclinical studies.Front Neurol. 2025 Jun 18;16:1532056. doi: 10.3389/fneur.2025.1532056. eCollection 2025. Front Neurol. 2025. PMID: 40606124 Free PMC article. Review.
Cited by
-
Roles of Ion Channels in Oligodendrocyte Precursor Cells: From Physiology to Pathology.Int J Mol Sci. 2025 Jul 29;26(15):7336. doi: 10.3390/ijms26157336. Int J Mol Sci. 2025. PMID: 40806469 Free PMC article. Review.
-
Hypoxic Neuroinflammation in the Pathogenesis of Multiple Sclerosis.Brain Sci. 2025 Feb 26;15(3):248. doi: 10.3390/brainsci15030248. Brain Sci. 2025. PMID: 40149770 Free PMC article. Review.
References
-
- James SL, Theadom A, Ellenbogen RG, Bannick MS, Mountjoy-Venning WC, Lucchesi LR, et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):56–87. doi: 10.1016/S1474-4422(18)30415-0. - DOI - PMC - PubMed
-
- Lipton ML, Gellella E, Lo C, Gold T, Ardekani BA, Shifteh K, et al. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J Neurotrauma. 2008;25(11):1335–1342. doi: 10.1089/neu.2008.0547. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

