Treatment of elderly patients with non-ST-elevation myocardial infarction: the nationwide POPular age registry
- PMID: 37768542
- PMCID: PMC10834918
- DOI: 10.1007/s12471-023-01812-0
Treatment of elderly patients with non-ST-elevation myocardial infarction: the nationwide POPular age registry
Abstract
Objective: We describe the current treatment of elderly patients with non-ST-elevation myocardial infarction (NSTEMI) enrolled in a national registry.
Methods: The POPular AGE registry is a prospective, multicentre study of patients ≥ 75 years of age presenting with NSTEMI, performed in the Netherlands. Management was at the discretion of the treating physician. Cardiovascular events consisted of cardiovascular death, myocardial infarction and ischaemic stroke. Bleeding was classified according to the Bleeding Academic Research Consortium (BARC) criteria.
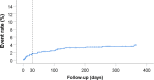
Results: A total of 646 patients were enrolled between August 2016 and May 2018. Median age was 81 (IQR 77-84) years and 58% were male. Overall, 75% underwent coronary angiography, 40% percutaneous coronary intervention, and 11% coronary artery bypass grafting, while 49.8% received pharmacological therapy only. At discharge, dual antiplatelet therapy (aspirin and P2Y12 inhibitor) was prescribed to 56.7%, and 27.4% received oral anticoagulation plus at least one antiplatelet agent. At 1‑year follow-up, cardiovascular death, myocardial infarction or stroke had occurred in 13.6% and major bleeding (BARC 3 and 5) in 3.9% of patients. The risk of both cardiovascular events and major bleeding was highest during the 1st month. However, cardiovascular risk was three times as high as bleeding risk in this elderly population, both after 1 month and after 1 year.
Conclusions: In this national registry of elderly patients with NSTEMI, the majority are treated according to current European Society of Cardiology guidelines. Both the cardiovascular and bleeding risk are highest during the 1st month after NSTEMI. However, the cardiovascular risk was three times as high as the bleeding risk.
Keywords: Antiplatelet therapy; Elderly; Non-ST-elevation myocardial infarction; Therapy.
© 2023. The Author(s).
Conflict of interest statement
M.E. Gimbel reports grants from AstraZeneca and from the St. Antonius research fund during the conduct of the study. A. van ’t Hof reports grants from Medtronic, Astra Zeneca and Abbott, outside the scope of the submitted work. J.M. ten Berg reports grants from AstraZeneca and from the St. Antonius research fund, personal fees from Boehringer Ingelheim, AstraZeneca, Bayer and Ferrer during the conduct of the study. D.R.P.P. Chan Pin Yin, W.W.A. van den Broek, R.S. Hermanides, F. Kauer, A.H. Tavenier, D. Schellings, S.L. Brinckman, S.H.K. The, M.G. Stoel, T.A.C.M. Heestermans, S. Rasoul, M.E. Emans, M. van de Wetering, P.F.M.M. van Bergen, R. Walhout, D. Nicastia, I. Aksoy, P. Knaapen, C.-J. Botman, A. Liem, C. de Nooijer, J. Peper and J.C. Kelder declare that they have no competing interests.
Figures
References
-
- Piccolo R, Magnani G, Ariotti S, et al. Ischaemic and bleeding outcomes in elderly patients undergoing a prolonged versus shortened duration of dual antiplatelet therapy after percutaneous coronary intervention: insights from the PRODIGY randomised trial. EuroIntervention. 2017;13(1):78–86. doi: 10.4244/EIJ-D-16-00497. - DOI - PubMed
LinkOut - more resources
Full Text Sources