Influenza, Tdap, and COVID-19 Vaccination Coverage and Hesitancy Among Pregnant Women - United States, April 2023
- PMID: 37768879
- PMCID: PMC10545430
- DOI: 10.15585/mmwr.mm7239a4
Influenza, Tdap, and COVID-19 Vaccination Coverage and Hesitancy Among Pregnant Women - United States, April 2023
Abstract
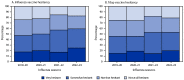
Influenza, tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap), and COVID-19 vaccines can reduce the risk for influenza, pertussis, and COVID-19 among pregnant women and their infants. To assess influenza, Tdap, and COVID-19 vaccination coverage among women pregnant during the 2022-23 influenza season, CDC analyzed data from an Internet panel survey conducted during March 28-April 16, 2023. Among 1,814 survey respondents who were pregnant at any time during October 2022-January 2023, 47.2% reported receiving influenza vaccine before or during their pregnancy. Among 776 respondents with a live birth by their survey date, 55.4% reported receiving Tdap vaccine during pregnancy. Among 1,252 women pregnant at the time of the survey, 27.3% reported receipt of a COVID-19 bivalent booster dose before or during the current pregnancy. Data from the same questions included in surveys conducted during influenza seasons 2019-20 through 2022-23 show that the proportion of pregnant women who reported being very hesitant about influenza and Tdap vaccinations during pregnancy increased from 2019-20 to 2022-23. Pregnant women who received a provider recommendation for vaccination were less hesitant about influenza and Tdap vaccines. Promotion of efforts to improve vaccination coverage among pregnant women, such as provider recommendation for vaccination and informative conversations with patients to address vaccine hesitancy, might reduce vaccine hesitancy and increase coverage with these important vaccines to protect mothers and their infants against severe respiratory diseases.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- Grohskopf LA, Blanton LH, Ferdinands JM, Chung JR, Broder KR, Talbot HK. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2023–24 influenza season. MMWR Recomm Rep 2023;72(No. RR-2):1–25. 10.15585/mmwr.rr7202a1 - DOI - PMC - PubMed
-
- Havers FP, Moro PL, Hunter P, Hariri S, Bernstein H. Use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2019. MMWR Morb Mortal Wkly Rep 2020;69:77–83. 10.15585/mmwr.mm6903a5 - DOI - PMC - PubMed
-
- CDC. Influenza (flu): flu, Tdap, and COVID-19 vaccination coverage among pregnant women—United States, April 2022. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://www.cdc.gov/flu/fluvaxview/pregnant-women-apr2022.htm
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

