Multiomics analyses reveal dynamic bioenergetic pathways and functional remodeling of the heart during intermittent fasting
- PMID: 37769126
- PMCID: PMC10538958
- DOI: 10.7554/eLife.89214
Multiomics analyses reveal dynamic bioenergetic pathways and functional remodeling of the heart during intermittent fasting
Abstract
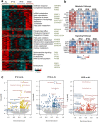
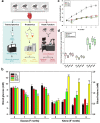
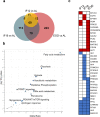
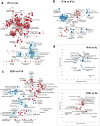
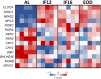
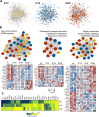
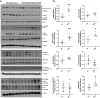
Intermittent fasting (IF) has been shown to reduce cardiovascular risk factors in both animals and humans, and can protect the heart against ischemic injury in models of myocardial infarction. However, the underlying molecular mechanisms behind these effects remain unclear. To shed light on the molecular and cellular adaptations of the heart to IF, we conducted comprehensive system-wide analyses of the proteome, phosphoproteome, and transcriptome, followed by functional analysis. Using advanced mass spectrometry, we profiled the proteome and phosphoproteome of heart tissues obtained from mice that were maintained on daily 12- or 16 hr fasting, every-other-day fasting, or ad libitum control feeding regimens for 6 months. We also performed RNA sequencing to evaluate whether the observed molecular responses to IF occur at the transcriptional or post-transcriptional levels. Our analyses revealed that IF significantly affected pathways that regulate cyclic GMP signaling, lipid and amino acid metabolism, cell adhesion, cell death, and inflammation. Furthermore, we found that the impact of IF on different metabolic processes varied depending on the length of the fasting regimen. Short IF regimens showed a higher correlation of pathway alteration, while longer IF regimens had an inverse correlation of metabolic processes such as fatty acid oxidation and immune processes. Additionally, functional echocardiographic analyses demonstrated that IF enhances stress-induced cardiac performance. Our systematic multi-omics study provides a molecular framework for understanding how IF impacts the heart's function and its vulnerability to injury and disease.
Keywords: RNA sequencing; heart; intermittent fasting; medicine; metabolism; mouse; phosphoproteomics; proteomics; regenerative medicine; stem cells.
© 2023, Arumugam, Alli-Shaik et al.
Conflict of interest statement
TA, AA, EL, SS, LP, VR, YC, YC, JK, JK, HS, DH, CR, DF, DM, GN, SB, KM, MG, GD, CS, BK, RS, MM, DJ, JG No competing interests declared
Figures










Update of
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

