Targeted Molecular Profiling of Circulating Cell-Free DNA in Patients With Advanced Hepatocellular Carcinoma
- PMID: 37769223
- PMCID: PMC10581608
- DOI: 10.1200/PO.23.00272
Targeted Molecular Profiling of Circulating Cell-Free DNA in Patients With Advanced Hepatocellular Carcinoma
Abstract
Purpose: Next-generation sequencing (NGS) of tumor-derived, circulating cell-free DNA (cfDNA) may aid in diagnosis, prognostication, and treatment of patients with hepatocellular carcinoma (HCC). The operating characteristics of cfDNA mutational profiling must be determined before routine clinical implementation.
Methods: This was a single-center, retrospective study with the primary objective of defining genomic alterations in circulating cfDNA along with plasma-tissue genotype agreement between NGS of matched tumor samples in patients with advanced HCC. cfDNA was analyzed using a clinically validated 129-gene NGS assay; matched tissue-based NGS was analyzed with a US Food and Drug Administration-authorized NGS tumor assay.
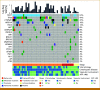
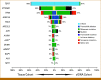
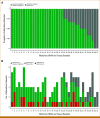
Results: Fifty-three plasma samples from 51 patients with histologically confirmed HCC underwent NGS-based cfDNA analysis. Genomic alterations were detected in 92.2% of patients, with the most commonly mutated genes including TERT promoter (57%), TP53 (47%), CTNNB1 (37%), ARID1A (18%), and TSC2 (14%). In total, 37 (73%) patients underwent paired tumor NGS, and concordance was high for mutations observed in patient-matched plasma samples: TERT (83%), TP53 (94%), CTNNB1 (92%), ARID1A (100%), and TSC2 (71%). In 10 (27%) of 37 tumor-plasma samples, alterations were detected by cfDNA analysis that were not detected in the patient-matched tumors. Potentially actionable mutations were identified in 37% of all cases including oncogenic/likely oncogenic alterations in TSC1/2 (18%), BRCA1/2 (8%), and PIK3CA (8%). Higher average variant allele fraction was associated with elevated alpha-fetoprotein, increased tumor volume, and no previous systemic therapy, but did not correlate with overall survival in treatment-naïve patients.
Conclusion: Tumor mutation profiling of cfDNA in HCC represents an alternative to tissue-based genomic profiling, given the high degree of tumor-plasma NGS concordance; however, genotyping of both blood and tumor may be required to detect all clinically actionable genomic alterations.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
-
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. - PubMed
-
- Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 10.1056/EVIDoa2100070 - DOI - PubMed
-
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

