Neuromelanin accumulation drives endogenous synucleinopathy in non-human primates
- PMID: 37769648
- PMCID: PMC10689915
- DOI: 10.1093/brain/awad331
Neuromelanin accumulation drives endogenous synucleinopathy in non-human primates
Abstract
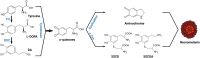
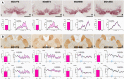
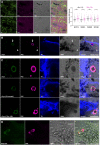
Although neuromelanin is a dark pigment characteristic of dopaminergic neurons in the human substantia nigra pars compacta, its potential role in the pathogenesis of Parkinson's disease (PD) has often been neglected since most commonly used laboratory animals lack neuromelanin. Here we took advantage of adeno-associated viral vectors encoding the human tyrosinase gene for triggering a time-dependent neuromelanin accumulation within substantia nigra pars compacta dopaminergic neurons in macaques up to similar levels of pigmentation as observed in elderly humans. Furthermore, neuromelanin accumulation induced an endogenous synucleinopathy mimicking intracellular inclusions typically observed in PD together with a progressive degeneration of neuromelanin-expressing dopaminergic neurons. Moreover, Lewy body-like intracellular inclusions were observed in cortical areas of the frontal lobe receiving dopaminergic innervation, supporting a circuit-specific anterograde spread of endogenous synucleinopathy by permissive trans-synaptic templating. In summary, the conducted strategy resulted in the development and characterization of a new macaque model of PD matching the known neuropathology of this disorder with unprecedented accuracy. Most importantly, evidence is provided showing that intracellular aggregation of endogenous α-synuclein is triggered by neuromelanin accumulation, therefore any therapeutic approach intended to decrease neuromelanin levels may provide appealing choices for the successful implementation of novel PD therapeutics.
Keywords: Lewy bodies; alpha-synuclein; marinesco bodies; prion-like spread; tyrosinase.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures




Comment in
-
Neuromelanin as a nidus for neurodegeneration.Brain. 2023 Dec 1;146(12):4794-4795. doi: 10.1093/brain/awad385. Brain. 2023. PMID: 37967242 Free PMC article.
References
-
- Fedorow H, Tribl F, Halliday G, Gerlanch M, Riederer P, Double KL. Neuromelanin in human dopamine neurons: Comparison with peripheral melanins and relevance to Parkinson’s disease. Prog Neurobiol. 2005;75:109–124. - PubMed
-
- Fedorow H, Halliday GM, Rickert CH, Gerlach M, Riederer P, Double KL. Evidence for specific phases in the development of human neuromelanin. Neurobiol Aging 2006;27:506–512. - PubMed
-
- Hirsch EC, Graybiel AM, Agid Y. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature 1988;334:345–348. - PubMed
-
- Hirsch EC, Graybiel AM, Agid Y. Selective vulnerability of pigmented dopaminergic neurons in Parkinson’s disease. Acta Neurol Scand. 1989;126:19–22. - PubMed
-
- Kastner A, Hirsch EC, Lejeune O, Javoy-Agid F, Rascol O, Agid Y. Is the vulnerability of neurons in the substantia nigra of patients with Parkinson’s disease related to their neuromelanin content? J Neurochem. 1992;59:1080–1089. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

