Porous silicon embedded in a thermoresponsive hydrogel for intranasal delivery of lipophilic drugs to treat rhinosinusitis
- PMID: 37769816
- PMCID: PMC11484479
- DOI: 10.1016/j.jconrel.2023.09.045
Porous silicon embedded in a thermoresponsive hydrogel for intranasal delivery of lipophilic drugs to treat rhinosinusitis
Abstract
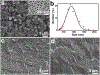
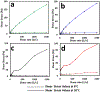
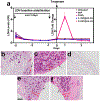
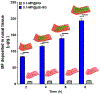
Intranasal delivery is the most preferred route of drug administration for treatment of a range of nasal conditions including chronic rhinosinusitis (CRS), caused by an infection and inflammation of the nasal mucosa. However, localised delivery of lipophilic drugs for persistent nasal inflammation is a challenge especially with traditional topical nasal sprays. In this study, a composite thermoresponsive hydrogel is developed and tuned to obtain desired rheological and physiochemical properties suitable for intranasal administration of lipophilic drugs. The composite is comprised of drug-loaded porous silicon (pSi) particles embedded in a poloxamer 407 (P407) hydrogel matrix. Mometasone Furoate (MF), a lipophilic corticosteroid (log P of 4.11), is used as the drug, which is loaded onto pSi particles at a loading capacity of 28 wt%. The MF-loaded pSi particles (MF@pSi) are incorporated into the P407-based thermoresponsive hydrogel (HG) matrix to form the composite hydrogel (MF@pSi-HG) with a final drug content ranging between 0.1 wt% to 0.5 wt%. Rheomechanical studies indicate that the MF@pSi component exerts a minimal impact on gelation temperature or strength of the hydrogel host. The in-vitro release of the MF payload from MF@pSi-HG shows a pronounced increase in the amount of drug released over 8 h (4.5 to 21-fold) in comparison to controls consisting of pure MF incorporated in hydrogel (MF@HG), indicating an improvement in kinetic solubility of MF upon loading into pSi. Ex-vivo toxicity studies conducted on human nasal mucosal tissue show no adverse effect from exposure to either pure HG or the MF@pSi-HG formulation, even at the highest drug content of 0.5 wt%. Experiments on human nasal mucosal tissue show the MF@pSi-HG formulation deposits a quantity of MF into the tissues within 8 h that is >19 times greater than the MF@HG control (194 ± 7 μg of MF/g of tissue vs. <10 μg of MF/g of tissue, respectively).
Keywords: Corticosteroid; Human nasal mucosal tissue; Mometasone furoate; Poloxamer 407; Stimuli-responsive hydrogels.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest MJS is a scientific founder (SF), member of the Board of Directors (BOD), Advisory Board (AB), Scientific Advisory Board (SAB), acts as a paid consultant (PC) or has an equity interest (EI) in the following: Aivocode, Inc. (AB, EI); Bejing ITEC Technologies (SAB, PC); Lisata Therapeutics (SF, SAB, EI); Illumina (EI), Matrix Technologies (EI); NanoVision Bio (SAB, EI); Quanterix (EI), Spinnaker Biosciences, Inc. (SF, BOD, EI); TruTag Technologies (SAB, EI); and Well-Healthcare Technologies (SAB, PC). Although one or more of the grants that supported this research has been identified for conflict of interest management based on the overall scope of the project and its potential benefit to the companies listed, the research findings included in this publication may not necessarily relate to their interests. The terms of these arrangements have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
Figures



References
-
- Hamilos DL, Chronic rhinosinusitis: epidemiology and medical management, J. Allergy Clin. Immunol 128 (2011) 693–707. - PubMed
-
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, Bousquet PJ, Brozek G, Bruno A, Dahlen SE, Forsberg B, Gunnbjornsdottir M, Kasper L, Kramer U, Kowalski ML, Lange B, Lundback B, Salagean E, Todo-Bom A, Tomassen P, Toskala E, van Drunen CM, Bousquet J, Zuberbier T, Jarvis D, Burney P, Chronic rhinosinusitis in European underestimated disease. A GA2LEN study, Allergy 66 (2011) 1216–1223. - PubMed
-
- Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, Brook I, Ashok Kumar K, Kramper M, Orlandi RR, Palmer JN, Patel ZM, Peters A, Walsh SA, Corrigan MD, Clinical practice guideline (update): adult sinusitis, Otolaryngol. Head Neck Surg 152 (2015) 1–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

