Quantitative Raman chemical imaging of intracellular drug-membrane aggregates and small molecule drug precipitates in cytoplasmic organelles
- PMID: 37769851
- PMCID: PMC10841539
- DOI: 10.1016/j.addr.2023.115107
Quantitative Raman chemical imaging of intracellular drug-membrane aggregates and small molecule drug precipitates in cytoplasmic organelles
Abstract
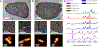
Raman confocal microscopes have been used to visualize the distribution of small molecule drugs within different subcellular compartments. This visualization allows the discovery, characterization, and detailed analysis of the molecular transport phenomena underpinning the Volume of Distribution - a key parameter governing the systemic pharmacokinetics of small molecule drugs. In the specific case of lipophilic small molecules with large Volumes of Distribution, chemical imaging studies using Raman confocal microscopes have revealed how weakly basic, poorly soluble drug molecules can accumulate inside cells by forming stable, supramolecular complexes in association with cytoplasmic membranes or by precipitating out within organelles. To study the self-assembly and function of the resulting intracellular drug inclusions, Raman chemical imaging methods have been developed to measure and map the mass, concentration, and ionization state of drug molecules at a microscopic, subcellular level. Beyond the field of drug delivery, Raman chemical imaging techniques relevant to the study of microscopic drug precipitates and drug-lipid complexes which form inside cells are also being developed by researchers with seemingly unrelated scientific interests. Highlighting advances in data acquisition, calibration methods, and computational data management and analysis tools, this review will cover a decade of technological developments that enable the conversion of spectral signals obtained from Raman confocal microscopes into new discoveries and information about previously unknown, concentrative drug transport pathways driven by soluble-to-insoluble phase transitions occurring within the cytoplasmic organelles of eukaryotic cells.
Keywords: Chemical imaging; Drug delivery; Drug targeting; Drug transport; Pharmacokinetics; Raman confocal microscopy.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: GRR owns stock in various companies in the pharmaceutical, biotechnology, and Raman microscopy space.
Figures






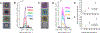



Similar articles
-
Molecular imaging of intracellular drug-membrane aggregate formation.Mol Pharm. 2011 Oct 3;8(5):1742-9. doi: 10.1021/mp200101b. Epub 2011 Aug 12. Mol Pharm. 2011. PMID: 21800872 Free PMC article.
-
The subcellular distribution of small molecules: from pharmacokinetics to synthetic biology.Mol Pharm. 2011 Oct 3;8(5):1619-28. doi: 10.1021/mp200092v. Epub 2011 Aug 15. Mol Pharm. 2011. PMID: 21805990 Free PMC article. Review.
-
Quantitative Drug Dynamics Visualized by Alkyne-Tagged Plasmonic-Enhanced Raman Microscopy.ACS Nano. 2020 Nov 24;14(11):15032-15041. doi: 10.1021/acsnano.0c05010. Epub 2020 Oct 20. ACS Nano. 2020. PMID: 33079538
-
Visualizing Bioactive Small Molecules by Alkyne Tagging and Slit-Scanning Raman Microscopy.Methods Mol Biol. 2019;1888:99-114. doi: 10.1007/978-1-4939-8891-4_5. Methods Mol Biol. 2019. PMID: 30519942
-
Raman Imaging Reveals Insights into Membrane Phase Biophysics in Cells.J Phys Chem B. 2023 Jul 20;127(28):6233-6240. doi: 10.1021/acs.jpcb.3c03125. Epub 2023 Jul 11. J Phys Chem B. 2023. PMID: 37431772 Free PMC article. Review.
Cited by
-
Advancing precision cancer immunotherapy drug development, administration, and response prediction with AI-enabled Raman spectroscopy.Front Immunol. 2025 Jan 9;15:1520860. doi: 10.3389/fimmu.2024.1520860. eCollection 2024. Front Immunol. 2025. PMID: 39850874 Free PMC article. Review.
References
-
- Flynn GL, Roberts MS, Physical and Biophysical Foundations of Pharmacy Practice: Issues in Drug Delivery, Michigan Publishing2015.
-
- Rosania GR, Thurber GM, Quantitative Analysis of Cellular Drug Transport, Disposition, and Delivery, Springer US2021.
-
- Chu X, Korzekwa K, Elsby R, Fenner K, Galetin A, Lai Y, Matsson P, Moss A, Nagar S, Rosania GR, Bai JP, Polli JW, Sugiyama Y, Brouwer KL, International Transporter C, Intracellular drug concentrations and transporters: measurement, modeling, and implications for the liver, Clin Pharmacol Ther, 94 (2013) 126–141. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

