Biomolecular condensates modulate membrane lipid packing and hydration
- PMID: 37770422
- PMCID: PMC10539446
- DOI: 10.1038/s41467-023-41709-5
Biomolecular condensates modulate membrane lipid packing and hydration
Abstract
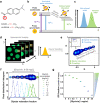
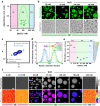
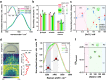
Membrane wetting by biomolecular condensates recently emerged as a key phenomenon in cell biology, playing an important role in a diverse range of processes across different organisms. However, an understanding of the molecular mechanisms behind condensate formation and interaction with lipid membranes is still missing. To study this, we exploited the properties of the dyes ACDAN and LAURDAN as nano-environmental sensors in combination with phasor analysis of hyperspectral and lifetime imaging microscopy. Using glycinin as a model condensate-forming protein and giant vesicles as model membranes, we obtained vital information on the process of condensate formation and membrane wetting. Our results reveal that glycinin condensates display differences in water dynamics when changing the salinity of the medium as a consequence of rearrangements in the secondary structure of the protein. Remarkably, analysis of membrane-condensates interaction with protein as well as polymer condensates indicated a correlation between increased wetting affinity and enhanced lipid packing. This is demonstrated by a decrease in the dipolar relaxation of water across all membrane-condensate systems, suggesting a general mechanism to tune membrane packing by condensate wetting.
© 2023. Springer Nature Limited.
Conflict of interest statement
All authors declare no competing interests.
Figures





Similar articles
-
Photoswitchable Endocytosis of Biomolecular Condensates in Giant Vesicles.Adv Sci (Weinh). 2024 Jun;11(23):e2309864. doi: 10.1002/advs.202309864. Epub 2024 Apr 6. Adv Sci (Weinh). 2024. PMID: 38582523 Free PMC article.
-
Lipid packing and cholesterol content regulate membrane wetting and remodeling by biomolecular condensates.Nat Commun. 2025 Mar 20;16(1):2756. doi: 10.1038/s41467-025-57985-2. Nat Commun. 2025. PMID: 40113768 Free PMC article.
-
Wetting and complex remodeling of membranes by biomolecular condensates.Nat Commun. 2023 May 22;14(1):2809. doi: 10.1038/s41467-023-37955-2. Nat Commun. 2023. PMID: 37217523 Free PMC article.
-
Biomolecular Condensates in Contact with Membranes.Annu Rev Biophys. 2024 Jul;53(1):319-341. doi: 10.1146/annurev-biophys-030722-121518. Epub 2024 Jun 28. Annu Rev Biophys. 2024. PMID: 38360555 Review.
-
Hitting the mark: Localization of mRNA and biomolecular condensates in health and disease.Wiley Interdiscip Rev RNA. 2023 Nov-Dec;14(6):e1807. doi: 10.1002/wrna.1807. Epub 2023 Jul 2. Wiley Interdiscip Rev RNA. 2023. PMID: 37393916 Free PMC article. Review.
Cited by
-
Regulating Biocondensates within Synthetic Cells via Segregative Phase Separation.ACS Nano. 2025 Jun 10;19(22):20550-20563. doi: 10.1021/acsnano.4c18971. Epub 2025 Apr 28. ACS Nano. 2025. PMID: 40293809 Free PMC article.
-
Spectral phasor imaging on a commercial confocal microscope without a spectral detector.Sci Rep. 2025 Aug 26;15(1):31431. doi: 10.1038/s41598-025-15637-x. Sci Rep. 2025. PMID: 40858664 Free PMC article.
-
Fluorescence lifetime sorting reveals tunable enzyme interactions within cytoplasmic condensates.J Cell Biol. 2025 Jan 6;224(1):e202311105. doi: 10.1083/jcb.202311105. Epub 2024 Oct 14. J Cell Biol. 2025. PMID: 39400294 Free PMC article.
-
Biomolecular Condensates can Induce Local Membrane Potentials.bioRxiv [Preprint]. 2024 Dec 27:2024.12.27.630407. doi: 10.1101/2024.12.27.630407. bioRxiv. 2024. PMID: 39763935 Free PMC article. Preprint.
-
Photoswitchable Endocytosis of Biomolecular Condensates in Giant Vesicles.Adv Sci (Weinh). 2024 Jun;11(23):e2309864. doi: 10.1002/advs.202309864. Epub 2024 Apr 6. Adv Sci (Weinh). 2024. PMID: 38582523 Free PMC article.
References
-
- Alberti S, Hyman AA. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 2021;22:196–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

