An amide to thioamide substitution improves the permeability and bioavailability of macrocyclic peptides
- PMID: 37770425
- PMCID: PMC10539501
- DOI: 10.1038/s41467-023-41748-y
An amide to thioamide substitution improves the permeability and bioavailability of macrocyclic peptides
Abstract
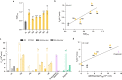
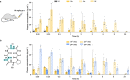
Solvent shielding of the amide hydrogen bond donor (NH groups) through chemical modification or conformational control has been successfully utilized to impart membrane permeability to macrocyclic peptides. We demonstrate that passive membrane permeability can also be conferred by masking the amide hydrogen bond acceptor (>C = O) through a thioamide substitution (>C = S). The membrane permeability is a consequence of the lower desolvation penalty of the macrocycle resulting from a concerted effect of conformational restriction, local desolvation of the thioamide bond, and solvent shielding of the amide NH groups. The enhanced permeability and metabolic stability on thioamidation improve the bioavailability of a macrocyclic peptide composed of hydrophobic amino acids when administered through the oral route in rats. Thioamidation of a bioactive macrocyclic peptide composed of polar amino acids results in analogs with longer duration of action in rats when delivered subcutaneously. These results highlight the potential of O to S substitution as a stable backbone modification in improving the pharmacological properties of peptide macrocycles.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures



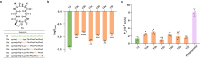

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

