A virus-like particle-based bivalent PCSK9 vaccine lowers LDL-cholesterol levels in non-human primates
- PMID: 37770440
- PMCID: PMC10539315
- DOI: 10.1038/s41541-023-00743-6
A virus-like particle-based bivalent PCSK9 vaccine lowers LDL-cholesterol levels in non-human primates
Abstract
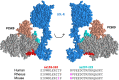
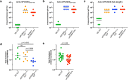
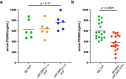
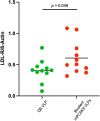
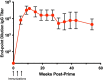
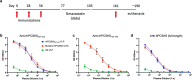
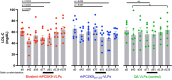
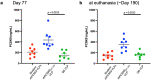
Elevated low-density lipoprotein cholesterol (LDL-C) is an important risk factor in the development of atherosclerotic cardiovascular disease (ASCVD). Inhibitors of proprotein convertase subtilisin/kexin type 9 (PCSK9), a negative regulator of LDL-C metabolism, have emerged as promising approaches for reducing elevated LDL-C levels. Here, we evaluated the cholesterol-lowering efficacy of virus-like particle (VLP) based vaccines that target epitopes found within the LDL receptor (LDL-R) binding domain of PCSK9. In both mice and non-human primates, a bivalent VLP vaccine targeting two distinct epitopes on PCSK9 elicited strong and durable antibody responses and lowered cholesterol levels. In macaques, a VLP vaccine targeting a single PCSK9 epitope was only effective at lowering LDL-C levels in combination with statins, whereas immunization with the bivalent vaccine lowered LDL-C without requiring statin co-administration. These data highlight the efficacy of an alternative, vaccine-based approach for lowering LDL-C.
© 2023. Springer Nature Limited.
Conflict of interest statement
B.C. has an equity stake in Metaphore Biotechnologies. A.F. is currently an employee of Moderna, Inc. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Update of
-
A Virus-like particle-based bivalent PCSK9 vaccine lowers LDL-cholesterol levels in Non-Human Primates.bioRxiv [Preprint]. 2023 May 15:2023.05.15.540560. doi: 10.1101/2023.05.15.540560. bioRxiv. 2023. Update in: NPJ Vaccines. 2023 Sep 28;8(1):142. doi: 10.1038/s41541-023-00743-6. PMID: 37292981 Free PMC article. Updated. Preprint.
Similar articles
-
A Virus-like particle-based bivalent PCSK9 vaccine lowers LDL-cholesterol levels in Non-Human Primates.bioRxiv [Preprint]. 2023 May 15:2023.05.15.540560. doi: 10.1101/2023.05.15.540560. bioRxiv. 2023. Update in: NPJ Vaccines. 2023 Sep 28;8(1):142. doi: 10.1038/s41541-023-00743-6. PMID: 37292981 Free PMC article. Updated. Preprint.
-
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors: Present perspectives and future horizons.Nutr Metab Cardiovasc Dis. 2016 Oct;26(10):853-62. doi: 10.1016/j.numecd.2016.05.006. Epub 2016 May 30. Nutr Metab Cardiovasc Dis. 2016. PMID: 27352986 Review.
-
Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis.J Am Coll Cardiol. 2013 Oct 15;62(16):1401-8. doi: 10.1016/j.jacc.2013.07.056. Epub 2013 Aug 21. J Am Coll Cardiol. 2013. PMID: 23973703 Review.
-
A cholesterol-lowering VLP vaccine that targets PCSK9.Vaccine. 2015 Oct 26;33(43):5747-5755. doi: 10.1016/j.vaccine.2015.09.044. Epub 2015 Sep 26. Vaccine. 2015. PMID: 26413878 Free PMC article.
-
An anti-PCSK9 antibody reduces LDL-cholesterol on top of a statin and suppresses hepatocyte SREBP-regulated genes.Int J Biol Sci. 2012;8(3):310-27. doi: 10.7150/ijbs.3524. Epub 2012 Feb 9. Int J Biol Sci. 2012. PMID: 22355267 Free PMC article.
Cited by
-
The Hurdle of Access to Emerging Therapies and Potential Solutions in the Management of Dyslipidemias.J Clin Med. 2024 Jul 16;13(14):4160. doi: 10.3390/jcm13144160. J Clin Med. 2024. PMID: 39064199 Free PMC article. Review.
-
Targeting of phosphorylated tau at threonine 181 by a Qβ virus-like particle vaccine is safe, highly immunogenic, and reduces disease severity in mice and rhesus macaques.Alzheimers Dement. 2025 Mar;21(3):e70101. doi: 10.1002/alz.70101. Alzheimers Dement. 2025. PMID: 40145301 Free PMC article.
-
Dyslipidemia: A Narrative Review on Pharmacotherapy.Pharmaceuticals (Basel). 2024 Feb 23;17(3):289. doi: 10.3390/ph17030289. Pharmaceuticals (Basel). 2024. PMID: 38543075 Free PMC article. Review.
-
Recent advances in immunotherapy targeting CETP proteins for atherosclerosis prevention.Hum Vaccin Immunother. 2025 Dec;21(1):2462466. doi: 10.1080/21645515.2025.2462466. Epub 2025 Feb 5. Hum Vaccin Immunother. 2025. PMID: 39907207 Free PMC article. Review.
-
Cholesterol reduction by immunization with a PCSK9 mimic.Cell Rep. 2024 Jun 25;43(6):114285. doi: 10.1016/j.celrep.2024.114285. Epub 2024 May 30. Cell Rep. 2024. PMID: 38819987 Free PMC article.
References
-
- 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis290, 140–205 (2019). - PubMed
-
- Boren J, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020;41:2313–2330. doi: 10.1093/eurheartj/ehz962. - DOI - PMC - PubMed
-
- Grundy SM, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1046–e1081. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

