Diminishing of Helicobacter pylori adhesion to Cavia porcellus gastric epithelial cells by BCG vaccine mycobacteria
- PMID: 37770504
- PMCID: PMC10539345
- DOI: 10.1038/s41598-023-43571-3
Diminishing of Helicobacter pylori adhesion to Cavia porcellus gastric epithelial cells by BCG vaccine mycobacteria
Abstract
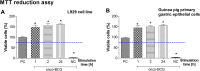
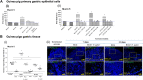
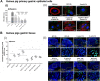
Mycobacterium bovis onco-BCG bacilli used in immunotherapy of bladder cancer are candidates for training of immune cells towards microbial pathogens. Increasing antibiotic resistance of gastric pathogen Helicobacter pylori (Hp) prompts the search for new anti-Hp and immunomodulatory formulations. Colonization of gastric mucosa by Hp through mucin 5 AC (MUC5AC) ligands could potentially be a therapeutic target. The aim of this study was to examine the ability of onco-BCG mycobacteria to reduce Hp adhesion to gastric epithelial cells using Cavia porcellus model. Animals were inoculated per os with 0.85% NaCl, Hp alone, onco-BCG alone or with onco-BCG and Hp. After 7/28 days Mucin5AC and Hp binding to gastric epithelium were assessed in gastric tissue specimens by staining with anti-Mucin5AC and anti-Hp antibodies, respectively, both fluorescently labeled. Primary gastric epithelial cells were treated ex vivo with live Hp or Hp surface antigens (glycine extract or lipopolysaccharide) alone or with onco-BCG. In such cells MUC5AC and Hp binding were determined as above. Mycobacteria reduced the amount of MUC5AC animals infected with Hp and in gastric epithelial cells pulsed in vitro with Hp components. Decrease of MUC5AC driven in cell cultures in vitro and in gastric tissue exposed ex vivo to mycobacteria was related to diminished adhesion of H. pylori bacilli. Vaccine mycobacteria by diminishing the amount of MUC5AC in gastric epithelial cells may reduce Hp adhesion.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Spray-dried pH-sensitive chitosan microparticles loaded with Mycobacterium bovis BCG intended for supporting treatment of Helicobacter pylori infection.Sci Rep. 2024 Feb 27;14(1):4747. doi: 10.1038/s41598-024-55353-6. Sci Rep. 2024. PMID: 38413775 Free PMC article.
-
Mycobacterium bovis BCG reverses deleterious effects of H. pylori components towards gastric barrier cells in vitro.Biomed Pharmacother. 2024 Sep;178:117193. doi: 10.1016/j.biopha.2024.117193. Epub 2024 Jul 26. Biomed Pharmacother. 2024. PMID: 39067167
-
Upregulation of MUC5AC production and deposition of LEWIS determinants by HELICOBACTER PYLORI facilitate gastric tissue colonization and the maintenance of infection.J Biomed Sci. 2019 Mar 6;26(1):23. doi: 10.1186/s12929-019-0515-z. J Biomed Sci. 2019. PMID: 30841890 Free PMC article.
-
[The role of gastric mucins in interactions with Helicobacter pylori].Postepy Hig Med Dosw (Online). 2012 Jan 30;66:60-6. Postepy Hig Med Dosw (Online). 2012. PMID: 22371407 Review. Polish.
-
Helicobacter pylori adhesion to gastric epithelial cells is mediated by glycan receptors.Braz J Med Biol Res. 2010 Jul;43(7):611-8. doi: 10.1590/s0100-879x2010007500049. Epub 2010 Jun 7. Braz J Med Biol Res. 2010. PMID: 20521012 Review.
Cited by
-
Trained Immunity and Trained Tolerance: The Case of Helicobacter pylori Infection.Int J Mol Sci. 2024 May 28;25(11):5856. doi: 10.3390/ijms25115856. Int J Mol Sci. 2024. PMID: 38892046 Free PMC article. Review.
-
Terpene-Based Eutectic Solvent Microdroplets: A Strategy to Combat Antibiotic-Resistant Helicobacter pylori.Langmuir. 2025 Jun 17;41(23):14915-14924. doi: 10.1021/acs.langmuir.5c01094. Epub 2025 Jun 3. Langmuir. 2025. PMID: 40458947 Free PMC article.
-
Spray-dried pH-sensitive chitosan microparticles loaded with Mycobacterium bovis BCG intended for supporting treatment of Helicobacter pylori infection.Sci Rep. 2024 Feb 27;14(1):4747. doi: 10.1038/s41598-024-55353-6. Sci Rep. 2024. PMID: 38413775 Free PMC article.
-
Dynamics of Bacteria-Mucus Interactions: Quantitative Assessment of MUC5AC and Helicobacter pylori Adhesion to the Gastric Tissue on the Model of Cavia porcellus.Methods Mol Biol. 2025;2942:23-33. doi: 10.1007/978-1-0716-4627-4_2. Methods Mol Biol. 2025. PMID: 40498303
References
-
- Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–1275. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

