Regulators of mitonuclear balance link mitochondrial metabolism to mtDNA expression
- PMID: 37770567
- PMCID: PMC11370000
- DOI: 10.1038/s41556-023-01244-3
Regulators of mitonuclear balance link mitochondrial metabolism to mtDNA expression
Abstract
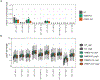
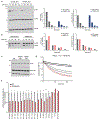
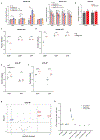
Mitochondrial oxidative phosphorylation (OXPHOS) complexes are assembled from proteins encoded by both nuclear and mitochondrial DNA. These dual-origin enzymes pose a complex gene regulatory challenge for cells requiring coordinated gene expression across organelles. To identify genes involved in dual-origin protein complex synthesis, we performed fluorescence-activated cell-sorting-based genome-wide screens analysing mutant cells with unbalanced levels of mitochondrial- and nuclear-encoded subunits of Complex IV. We identified genes involved in OXPHOS biogenesis, including two uncharacterized genes: PREPL and NME6. We found that PREPL specifically impacts Complex IV biogenesis by acting at the intersection of mitochondrial lipid metabolism and protein synthesis, whereas NME6, an uncharacterized nucleoside diphosphate kinase, controls OXPHOS biogenesis through multiple mechanisms reliant on its NDPK domain. Firstly, NME6 forms a complex with RCC1L, which together perform nucleoside diphosphate kinase activity to maintain local mitochondrial pyrimidine triphosphate levels essential for mitochondrial RNA abundance. Secondly, NME6 modulates the activity of mitoribosome regulatory complexes, altering mitoribosome assembly and mitochondrial RNA pseudouridylation. Taken together, we propose that NME6 acts as a link between compartmentalized mitochondrial metabolites and mitochondrial gene expression.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Figures

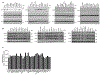




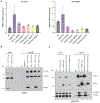


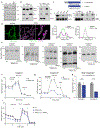

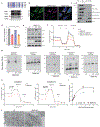
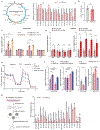

Update of
-
Genome-wide screens for mitonuclear co-regulators uncover links between compartmentalized metabolism and mitochondrial gene expression.bioRxiv [Preprint]. 2023 Feb 11:2023.02.11.528118. doi: 10.1101/2023.02.11.528118. bioRxiv. 2023. Update in: Nat Cell Biol. 2023 Nov;25(11):1575-1589. doi: 10.1038/s41556-023-01244-3. PMID: 36798306 Free PMC article. Updated. Preprint.
References
-
- Ryan MT & Hoogenraad NJ Mitochondrial-nuclear communications. Annu. Rev. Biochem 76, 701–722 (2007). - PubMed
-
- Mottis A, Herzig S & Auwerx J Mitocellular communication: Shaping health and disease. Science 366, 827–832 (2019). - PubMed
-
- Isaac RS, McShane E & Churchman LS The Multiple Levels of Mitonuclear Coregulation. Annu. Rev. Genet 52, 511–533 (2018). - PubMed
-
- Chan DC Mitochondria: dynamic organelles in disease, aging, and development. Cell 125, 1241–1252 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

