Efficacy of cell-free DNA methylation-based blood test for colorectal cancer screening in high-risk population: a prospective cohort study
- PMID: 37770864
- PMCID: PMC10538018
- DOI: 10.1186/s12943-023-01866-z
Efficacy of cell-free DNA methylation-based blood test for colorectal cancer screening in high-risk population: a prospective cohort study
Abstract
Background: Although colonoscopy is the standard screening test for colorectal cancer (CRC), its use is limited by a poor compliance rate, the need for extensive bowel preparation, and the risk of complications. As an alternative, an FDA-approved stool-based DNA test, Cologuard, has demonstrated satisfactory detection performance for CRC, but its compliance rate remains suboptimal, primarily attributable to individuals' reluctance to provide stool samples.
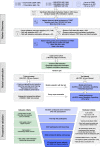
Methods: We developed a noninvasive blood-based CRC test, ColonSecure, based on cell-free DNA containing cancer-specific CpG island methylation patterns. We initially screened publicly available datasets for differentially methylated CpG sites in CRC with prediction potential. Subsequently, we performed two sequential bisulfite-free methylation sequencing on blood samples obtained from CRC patients and non-cancer controls. Through rigorous evaluation of each marker and machine learning-assisted feature selection, we identified 149 hypermethylated markers from over 193,000 CpG sites. These markers were then utilized to construct the ColonSecure model, enabling accurate CRC detection.
Results: We validated the efficacy of our cell-free DNA methylation-based blood test for CRC screening with 3493 high-risk individuals identified from 114,136 urban residents. The ColonSecure test identified 89 out of 103 CRC patients diagnosed by the follow-up colonoscopy, outperforming CEA, CRP, and CA19-9 (with a sensitivity of 86.4% compared to 45.6%, 39.8%, and 25.2% for CEA, CRP, and CA19-9 respectively; an AUROC of 0.956 compared to an AUROC of < 0.77 for other methods).
Conclusion: Our observations emphasize the potential of our multiple cfDNA methylation marker-based test for CRC screening in high-risk populations.
Keywords: Cell-free DNA; Colorectal cancer; Hypermethylation; Populational screening; Prospective validation.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
J.X., D.L., and Z.Z. are employees of LAMH. Q.L., Y.G., and D.L. are inventors on the patent related to the technologies described here (patent number ZL2022101881947).
Figures

References
-
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021. 10.1002/ijc.33588. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

