Sexual differences in locus coeruleus neurons and related behavior in C57BL/6J mice
- PMID: 37770907
- PMCID: PMC10540344
- DOI: 10.1186/s13293-023-00550-7
Sexual differences in locus coeruleus neurons and related behavior in C57BL/6J mice
Abstract
Background: In addition to social and cultural factors, sex differences in the central nervous system have a critical influence on behavior, although the neurobiology underlying these differences remains unclear. Interestingly, the Locus Coeruleus (LC), a noradrenergic nucleus that exhibits sexual dimorphism, integrates signals that are related to diverse activities, including emotions, cognition and pain. Therefore, we set-out to evaluate sex differences in behaviors related to LC nucleus, and subsequently, to assess the sex differences in LC morphology and function.
Methods: Female and male C57BL/6J mice were studied to explore the role of the LC in anxiety, depressive-like behavior, well-being, pain, and learning and memory. We also explored the number of noradrenergic LC cells, their somatodendritic volume, as well as the electrophysiological properties of LC neurons in each sex.
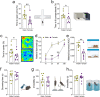
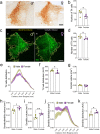
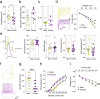
Results: While both male and female mice displayed similar depressive-like behavior, female mice exhibited more anxiety-related behaviors. Interestingly, females outperformed males in memory tasks that involved distinguishing objects with small differences and they also showed greater thermal pain sensitivity. Immunohistological analysis revealed that females had fewer noradrenergic cells yet they showed a larger dendritic volume than males. Patch clamp electrophysiology studies demonstrated that LC neurons in female mice had a lower capacitance and that they were more excitable than male LC neurons, albeit with similar action potential properties.
Conclusions: Overall, this study provides new insights into the sex differences related to LC nucleus and associated behaviors, which may explain the heightened emotional arousal response observed in females.
Keywords: Anxiety; Depression; Electrophysiology; Female; Learning and memory; Locus coeruleus; Noradrenaline; Pain; Patch clamp; Sex.
Plain language summary
Exploring sex differences in the brain is important to understand the impact of such differences in pathological conditions characterized by gender bias, as well as their therapeutic implications. In this manuscript, we examined sex differences in the mouse locus coeruleus (LC) and how this might affect related behaviours. The LC is a sexually dimorphic nucleus that integrates signals associated with attention, anxiety, stress, arousal, pain, memory and learning. Our findings reveal that female mice exhibit more intense anxiety-related behaviors but that they perform better than males in recognizing small differences between objects. Additionally, we found pronounced sex differences in the LC, which contained fewer noradrenergic cells in females, with a larger dendritic volume and displaying enhanced cell excitability. These differences in the LC, a nucleus that fulfils a pivotal role in stress and pain, could be important for understanding the higher prevalence of stress-related disorders in women, such as anxiety and depression, but also of chronic pain. Hence, it is clearly important to consider sex differences in both preclinical and clinical research studies that attempt to understand pathologies related to these phenomena.
© 2023. Society for Women's Health Research and BioMed Central Ltd.
Conflict of interest statement
The authors have no biomedical financial interests or potential competing interests to report.
Figures

Similar articles
-
Depressive-like behavior observed with a minimal loss of locus coeruleus (LC) neurons following administration of 6-hydroxydopamine is associated with electrophysiological changes and reversed with precursors of norepinephrine.Neuropharmacology. 2016 Feb;101:76-86. doi: 10.1016/j.neuropharm.2015.09.003. Epub 2015 Sep 8. Neuropharmacology. 2016. PMID: 26362360 Free PMC article.
-
Precise and Pervasive Phasic Bursting in Locus Coeruleus during Maternal Behavior in Mice.J Neurosci. 2022 Apr 6;42(14):2986-2999. doi: 10.1523/JNEUROSCI.0938-21.2022. Epub 2022 Mar 10. J Neurosci. 2022. PMID: 35273081 Free PMC article.
-
Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal.Physiol Behav. 2011 Jun 1;103(3-4):342-51. doi: 10.1016/j.physbeh.2011.02.037. Epub 2011 Mar 6. Physiol Behav. 2011. PMID: 21362438 Free PMC article.
-
Causes, consequences, and cures for neuroinflammation mediated via the locus coeruleus: noradrenergic signaling system.J Neurochem. 2016 Oct;139 Suppl 2:154-178. doi: 10.1111/jnc.13447. Epub 2016 Mar 10. J Neurochem. 2016. PMID: 26968403 Review.
-
Heterogeneous organization of Locus coeruleus: An intrinsic mechanism for functional complexity.Physiol Behav. 2023 Sep 1;268:114231. doi: 10.1016/j.physbeh.2023.114231. Epub 2023 May 10. Physiol Behav. 2023. PMID: 37172640 Review.
Cited by
-
Diversity of ancestral brainstem noradrenergic neurons across species and multiple biological factors.bioRxiv [Preprint]. 2024 Oct 16:2024.10.14.618224. doi: 10.1101/2024.10.14.618224. bioRxiv. 2024. PMID: 39464004 Free PMC article. Preprint.
-
Nerve injury induces pain hypersensitivity and anxiety-related behaviours and is associated with amygdala activation in male mice.PLoS One. 2025 May 7;20(5):e0323203. doi: 10.1371/journal.pone.0323203. eCollection 2025. PLoS One. 2025. PMID: 40334210 Free PMC article.
-
A Systematic Review and Meta-Analysis of Anxiety- and Depressive-Like Behaviors in Rodent Models of Neuropathic Pain.Biol Psychiatry Glob Open Sci. 2024 Aug 26;4(6):100388. doi: 10.1016/j.bpsgos.2024.100388. eCollection 2024 Nov. Biol Psychiatry Glob Open Sci. 2024. PMID: 39416657 Free PMC article.
-
Daily rhythms drive dynamism in sleep, oscillations and interneuron firing, while excitatory firing remains stable across 24 h.Eur J Neurosci. 2025 Jan;61(1):e16619. doi: 10.1111/ejn.16619. Epub 2024 Dec 11. Eur J Neurosci. 2025. PMID: 39663213 Free PMC article.
-
Photobiomodulation modulates mitochondrial energy metabolism and ameliorates neurological damage in an APP/PS1 mousmodel of Alzheimer's disease.Alzheimers Res Ther. 2025 Apr 5;17(1):72. doi: 10.1186/s13195-025-01714-w. Alzheimers Res Ther. 2025. PMID: 40188044 Free PMC article.
References
-
- Hamson DK, Roes MM, Galea LA. Sex hormones and cognition: neuroendocrine influences on memory and learning. Compr Physiol. 2016;6(3):1295–1337. - PubMed
-
- Angst J, Gamma A, Gastpar M, Lépine JP, Mendlewicz J, Tylee A, et al. Gender differences in depression. Epidemiological findings from the European DEPRES I and II studies. Eur Arch Psychiatry Clin Neurosci. 2002;252(5):201–9. - PubMed
-
- Bekker MH, van Mens-Verhulst J. Anxiety disorders: sex differences in prevalence, degree, and background, but gender-neutral treatment. Gend Med. 2007;4:S178–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

