Assessment of the diagnostic accuracy of Vibrasense compared to a biothesiometer and nerve conduction study for screening diabetic peripheral neuropathy
- PMID: 37770911
- PMCID: PMC10537102
- DOI: 10.1186/s13047-023-00667-3
Assessment of the diagnostic accuracy of Vibrasense compared to a biothesiometer and nerve conduction study for screening diabetic peripheral neuropathy
Abstract
Aims: Peripheral neuropathy is a common microvascular complication in diabetes and a risk factor for the development of diabetic foot ulcers and amputations. Vibrasense (Ayati Devices) is a handheld, battery-operated, rapid screening device for diabetic peripheral neuropathy (DPN) that works by quantifying vibration perception threshold (VPT). In this study, we compared Vibrasense against a biothesiometer and nerve conduction study for screening DPN.
Methods: A total of 562 subjects with type 2 diabetes mellitus underwent neuropathy assessments including clinical examination, 10-g monofilament test, VPT evaluation with Vibrasense and a standard biothesiometer. Those with an average VPT ≥ 15 V with Vibrasense were noted to have DPN. A subset of these patients (N = 61) underwent nerve conduction study (NCS). Diagnostic accuracy of Vibrasense was compared against a standard biothesiometer and abnormal NCS.
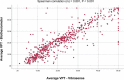
Results: Average VPTs measured with Vibrasense had a strong positive correlation with standard biothesiometer values (Spearman's correlation 0.891, P < 0.001). Vibrasense showed sensitivity and specificity of 87.89% and 86.81% compared to biothesiometer, and 82.14% and 78.79% compared to NCS, respectively.
Conclusions: Vibrasense demonstrated good diagnostic accuracy for detecting peripheral neuropathy in type 2 diabetes and can be an effective screening device in routine clinical settings.
Trial registration: Clinical trials registry of India (CTRI/2022/11/047002). Registered 3 November 2022. https://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=76167 .
Keywords: Biothesiometer; Diabetic peripheral neuropathy; Quantitative sensory testing; Type 2 diabetes mellitus; Vibration perception threshold.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Usefulness of the vibration perception thresholds measurement as a diagnostic method for diabetic peripheral neuropathy: Results from the Rio de Janeiro type 2 diabetes cohort study.J Diabetes Complications. 2018 Aug;32(8):770-776. doi: 10.1016/j.jdiacomp.2018.05.010. Epub 2018 Jun 18. J Diabetes Complications. 2018. PMID: 29950276
-
Quantitative vibration perception threshold in assessing diabetic polyneuropathy: Should the cut-off value be adjusted for Chinese individuals with type 2 diabetes?J Diabetes Investig. 2021 Sep;12(9):1663-1670. doi: 10.1111/jdi.13515. Epub 2021 Feb 20. J Diabetes Investig. 2021. PMID: 33512757 Free PMC article.
-
A comparison of the neurothesiometer and biothesiometer for measuring vibration perception in diabetic patients.Diabetes Res Clin Pract. 1993 May;20(2):129-31. doi: 10.1016/0168-8227(93)90006-q. Diabetes Res Clin Pract. 1993. PMID: 8375265 Clinical Trial.
-
Acetyl-L-carnitine for the treatment of diabetic peripheral neuropathy.Cochrane Database Syst Rev. 2019 Jun 15;6(6):CD011265. doi: 10.1002/14651858.CD011265.pub2. Cochrane Database Syst Rev. 2019. PMID: 31201734 Free PMC article.
-
Application of the Ipswich Touch Test for diabetic peripheral neuropathy screening: a systematic review and meta-analysis.BMJ Open. 2021 Oct 4;11(10):e046966. doi: 10.1136/bmjopen-2020-046966. BMJ Open. 2021. PMID: 34607858 Free PMC article.
Cited by
-
Artificial Intelligence Algorithm to Screen for Diabetic Neuropathy: A Pilot Study.Biomedicines. 2025 Apr 29;13(5):1075. doi: 10.3390/biomedicines13051075. Biomedicines. 2025. PMID: 40426905 Free PMC article.
-
Using biothesiometer, Neuropathy Symptom Score, and Neuropathy Disability Score for the early detection of peripheral neuropathy: A cross-sectional study.Qatar Med J. 2024 Jul 29;2024(3):24. doi: 10.5339/qmj.2024.24. eCollection 2024. Qatar Med J. 2024. PMID: 39131795 Free PMC article.
References
-
- Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, Lavery LA, Lemaster JW, Mills JL, Sr, Mueller MJ, Sheehan P, Wukich DK, American Diabetes Association; American Association of Clinical Endocrinologists Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679–85. doi: 10.2337/dc08-9021. - DOI - PMC - PubMed
-
- Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Fitridge R, Game F, Monteiro-Soares M, Senneville E. IWGDF Editorial Board. Practical guidelines on the prevention and management of diabetes-related foot disease (IWGDF 2023 update). Diabetes Metab Res Rev. 2023:e3657. 10.1002/dmrr.3657. - PubMed
-
- ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Gibbons CH, Giurini JM, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Sun JK, Gabbay RA, on behalf of the American Diabetes Association 12. Retinopathy, neuropathy, and foot care: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S203–S215. doi: 10.2337/dc23-S012. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical