Oxamate enhances the efficacy of CAR-T therapy against glioblastoma via suppressing ectonucleotidases and CCR8 lactylation
- PMID: 37770937
- PMCID: PMC10540361
- DOI: 10.1186/s13046-023-02815-w
Oxamate enhances the efficacy of CAR-T therapy against glioblastoma via suppressing ectonucleotidases and CCR8 lactylation
Abstract
Background: Chimeric antigen receptor (CAR)-T immunotherapy fails to treat solid tumors due in part to immunosuppressive microenvironment. Excess lactate produced by tumor glycolysis increases CAR-T immunosuppression. The mechanism of lactate inducing the formation of immunosuppressive microenvironment remains to be further explored.
Methods: Immunocyte subpopulations and molecular characteristics were analyzed in the orthotopic xenografts of nude mice using flow cytometry assay and immunohistochemical staining after oxamate, a lactate dehydrogenase A (LDHA) inhibitor, and control T or CAR-T cells injection alone or in combination. RT-qPCR, western blot, flow cytometry, immunofluorescence, luciferase reporter assay, chromatin immunoprecipitation and ELISA were performed to measure the effect of lactate on the regulation of CD39, CD73 and CCR8 in cultured glioma stem cells, CD4 + T cells or macrophages.
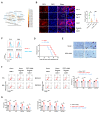
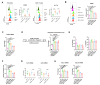
Results: Oxamate promoted immune activation of tumor-infiltrating CAR-T cells through altering the phenotypes of immune molecules and increasing regulatory T (Treg) cells infiltration in a glioblastoma mouse model. Lactate accumulation within cells upregulated CD39, CD73 and CCR8 expressions in both lactate-treated cells and glioma stem cells-co-cultured CD4 + T cells and macrophages, and intracellular lactate directly elevated the activities of these gene promotors through histone H3K18 lactylation.
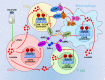
Conclusions: Utilizing lactate generation inhibitor not only reprogramed glucose metabolism of cancer stem cells, but also alleviated immunosuppression of tumor microenvironment and reduced tumor-infiltrating CAR-Treg cells, which may be a potential strategy to enhance CAR-T function in glioblastoma therapy.
Keywords: CAR-T; CCR8; Ectonucleotidases; Glioblastoma; Histone lactylation.
© 2023. Italian National Cancer Institute ‘Regina Elena’.
Conflict of interest statement
No potential conflicts of interest were evident or relevant in the context of this article, and thus there was nothing to disclose.
Figures




Similar articles
-
Suberanilohydroxamic acid (SAHA), a HDAC inhibitor, suppresses the effect of Treg cells by targeting the c-Myc/CCL1 pathway in glioma stem cells and improves PD-L1 blockade therapy.J Neurooncol. 2024 Jul;168(3):457-471. doi: 10.1007/s11060-024-04689-0. Epub 2024 Apr 23. J Neurooncol. 2024. PMID: 38652401
-
Enhanced radiation-induced immunogenic cell death activates chimeric antigen receptor T cells by targeting CD39 against glioblastoma.Cell Death Dis. 2022 Oct 16;13(10):875. doi: 10.1038/s41419-022-05319-1. Cell Death Dis. 2022. PMID: 36245000 Free PMC article.
-
Adoptive Transfer of IL13Rα2-Specific Chimeric Antigen Receptor T Cells Creates a Pro-inflammatory Environment in Glioblastoma.Mol Ther. 2018 Apr 4;26(4):986-995. doi: 10.1016/j.ymthe.2018.02.001. Epub 2018 Feb 8. Mol Ther. 2018. PMID: 29503195 Free PMC article.
-
Chimeric Antigen Receptor T Cells for Glioblastoma: Current Concepts, Challenges, and Future Perspectives.Neurology. 2021 Aug 3;97(5):218-230. doi: 10.1212/WNL.0000000000012193. Epub 2021 May 13. Neurology. 2021. PMID: 33986138 Review.
-
Challenges in the Treatment of Glioblastoma by Chimeric Antigen Receptor T-Cell Immunotherapy and Possible Solutions.Front Immunol. 2022 Jul 7;13:927132. doi: 10.3389/fimmu.2022.927132. eCollection 2022. Front Immunol. 2022. PMID: 35874698 Free PMC article. Review.
Cited by
-
Regulation of CD73 on NAD metabolism: Unravelling the interplay between tumour immunity and tumour metabolism.Cell Commun Signal. 2024 Aug 1;22(1):387. doi: 10.1186/s12964-024-01755-y. Cell Commun Signal. 2024. PMID: 39090604 Free PMC article. Review.
-
A rising star involved in tumour immunity: Lactylation.J Cell Mol Med. 2024 Oct;28(20):e70146. doi: 10.1111/jcmm.70146. J Cell Mol Med. 2024. PMID: 39417674 Free PMC article. Review.
-
Histone lactylation: from tumor lactate metabolism to epigenetic regulation.Int J Biol Sci. 2024 Mar 3;20(5):1833-1854. doi: 10.7150/ijbs.91492. eCollection 2024. Int J Biol Sci. 2024. PMID: 38481814 Free PMC article. Review.
-
Lactylation modification in cancer: mechanisms, functions, and therapeutic strategies.Exp Hematol Oncol. 2025 Mar 8;14(1):32. doi: 10.1186/s40164-025-00622-x. Exp Hematol Oncol. 2025. PMID: 40057816 Free PMC article. Review.
-
Emerging roles of lysine lactyltransferases and lactylation.Nat Cell Biol. 2025 Apr;27(4):563-574. doi: 10.1038/s41556-025-01635-8. Epub 2025 Apr 4. Nat Cell Biol. 2025. PMID: 40185947 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

