Genetic background influences arterial vasomotor function in male and female mice
- PMID: 37771071
- PMCID: PMC10539628
- DOI: 10.14814/phy2.15824
Genetic background influences arterial vasomotor function in male and female mice
Abstract
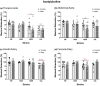
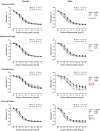
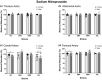
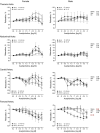
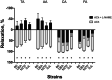
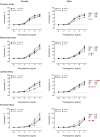
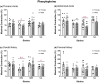
The purpose of this study was to assess the influence of genetic background and sex on nitric oxide (NO)-mediated vasomotor function in arteries from different vascular territories. Vasomotor function was assessed in thoracic aorta, abdominal aorta, carotid arteries, and femoral arteries from the following mouse strains: SJL/J, DBA/2J, NZW/LacJ, and C57BL/6J. Contractile responses were assessed using the α1-adrenergic receptor agonist phenylephrine (PE, 10-9 -10-5 M). Vasorelaxation responses were assessed by examining relaxation to an endothelium-dependent vasodilator acetylcholine (ACh, 10-9 -10-5 M) and an endothelium-independent vasodilator sodium nitroprusside (SNP, 10-9 -10-5 M). To evaluate the role of NO, relaxation responses to ACh and SNP were assessed in the absence or presence of a nitric oxide synthase inhibitor (N omega-nitro-l-arginine methyl ester hydrochloride: 10-4 M). Vasomotor responses to ACh and PE varied across strains and among the arteries tested with some strains exhibiting artery-specific impairment. Results indicated some concentration-response heterogeneity in response to ACh and SNP between vessels from females and males, but no significant differences in responses to PE. Collectively, these findings indicate that vasomotor responses vary by genetic background, sex, and artery type.
Keywords: aorta; carotid artery; endothelium; femoral artery; inbred mice; sex differences.
© 2023 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Celermajer, D. S. , Sorensen, K. E. , Spiegelhalter, D. J. , Georgakopoulos, D. , Robinson, J. , & Deanfield, J. E. (1994). Aging is associated with endothelial dysfunction in healthy men years before the age‐related decline in women. Journal of the American College of Cardiology, 24, 471–476. 10.1016/0735-1097(94)90305-0 - DOI - PubMed
-
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences. L. Erlbaum Associates.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

