Anti-virulence strategy of diaryl chalcogenide compounds against Candida albicans infection
- PMID: 37771181
- PMCID: PMC10549196
- DOI: 10.1080/21505594.2023.2265012
Anti-virulence strategy of diaryl chalcogenide compounds against Candida albicans infection
Abstract
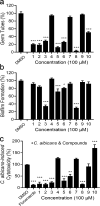
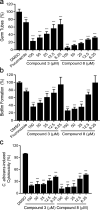
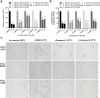
Candida albicans is an important opportunistic pathogenic fungus that frequently causes serious systemic infection in humans. Due to the vital roles of biofilm formation and the yeast-to-hypha transition in the infection process, we have selected a series of diaryl chalcogenides and tested their efficacy against C. albicans SC5314 pathogenicity by the inhibition of biofilm formation and the yeast-to-hypha transition. The compounds 5-sulfenylindole and 5-selenylindole were found to have excellent abilities to inhibit both biofilm formation and hyphal formation in C. albicans SC5314. Intriguingly, the two leading compounds also markedly attenuated C. albicans SC5314 virulence in human cell lines and mouse infection models at micromolar levels. Furthermore, our results showed that the presence of the compounds at 100 µM resulted in a marked decrease in the expression of genes involved in the cAMP-PKA and MAPK pathways in C. albicans SC5314. Intriguingly, the compounds 5-sulfenylindole and 5-selenylindole not only attenuated the cytotoxicity of Candida species strains but also showed excellent synergistic effects with antifungal agents against the clinical drug-resistant C. albicans strain HCH12. The compound 5-sulfenylindole showed an obvious advantage over fluconazole as it could also restore the composition and richness of the intestinal microbiota in mice infected by C. albicans. Together, these results suggest that diaryl chalcogenides can potentially be designed as novel clinical therapeutic agents against C. albicans infection. The diaryl chalcogenides of 5-sulfenylindole and 5-selenylindole discovered in this study can provide new direction for developing antifungal agents against C. albicans infection.
Keywords: Candida albicans; diaryl chalcogenide; gut microbiota; hyphal morphogenesis; virulence.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
