Preclinical assessment of a novel human antibody VH domain targeting mesothelin as an antibody-drug conjugate
- PMID: 37771390
- PMCID: PMC10522976
- DOI: 10.1016/j.omto.2023.09.002
Preclinical assessment of a novel human antibody VH domain targeting mesothelin as an antibody-drug conjugate
Abstract
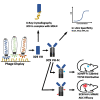
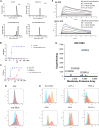
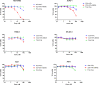
Mesothelin (MSLN) has been a validated tumor-associated antigen target for several solid tumors for over a decade, making it an attractive option for therapeutic interventions. Novel antibodies with high affinity and better therapeutic properties are needed. In the current study, we have isolated and characterized a novel heavy chain variable (VH) domain 3C9 from a large-size human immunoglobulin VH domain library. 3C9 exhibited high affinity (KD [dissociation constant] <3 nM) and binding specificity in a membrane proteome array (MPA). In a mouse xenograft model, 3C9 fused to human IgG1 Fc was detected at tumor sites as early as 8 h post-infusion and remained at the site for over 10 days. Furthermore, 3C9 fused to a human Fc domain drug conjugate effectively inhibited MSLN-positive tumor growth in a mouse xenograft model. The X-ray crystal structure of full-length MSLN in complex with 3C9 reveals interaction of the 3C9 domains with two distinctive residue patches on the MSLN surface. This newly discovered VH antibody domain has a high potential as a therapeutic candidate for MSLN-expressing cancers.
Keywords: ADC; MSLN; VH domain; in vivo distribution; library; structure.
© 2023 The Author(s).
Conflict of interest statement
Z.S., J.W.M., and D.S.D. are co-inventors of a patent filed by the University of Pittsburgh, related to 3C9 described in this paper.
Figures





References
-
- Hassan R., Remaley A.T., Sampson M.L., Zhang J., Cox D.D., Pingpank J., Alexander R., Willingham M., Pastan I., Onda M. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin. Cancer Res. 2006;12:447–453. doi: 10.1158/1078-0432.CCR-05-1477. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

